Discussion on Digital Therapy Layout (3)-Product Implementation
Discussion on DTx product specifications
Product specifications can be discussed from the DTx-Value-Assessment-Guide. Although the purpose of this guide is to provide health care delivery systems with a reference standard for evaluating the value of DTx, these references for value assessment actually express the specification needs of the product. How to open and plan.
1. Specification formulation
Using the general principles of medical product development requirements, products are classified from five major principles: product requirements, functional compliance, safety compliance, regulatory approval, and post-market tracking.
The main reason why post market follow up (Post market follow up) is adopted instead of post market surveillance (Post Market Surveillance) is that post market surveillance is the general direction that the quality system must implement, and for products such as DTx, after the quality system is launched, Under the supervision structure, the most important task is post-market tracking.
The 18 items of DTx Product Evaluation Considerations of DTx-Value-Assessment-Guide are used to classify Fig 1. For details of each item, please refer to DTx-Value-Assessment-Guide[Note 1]
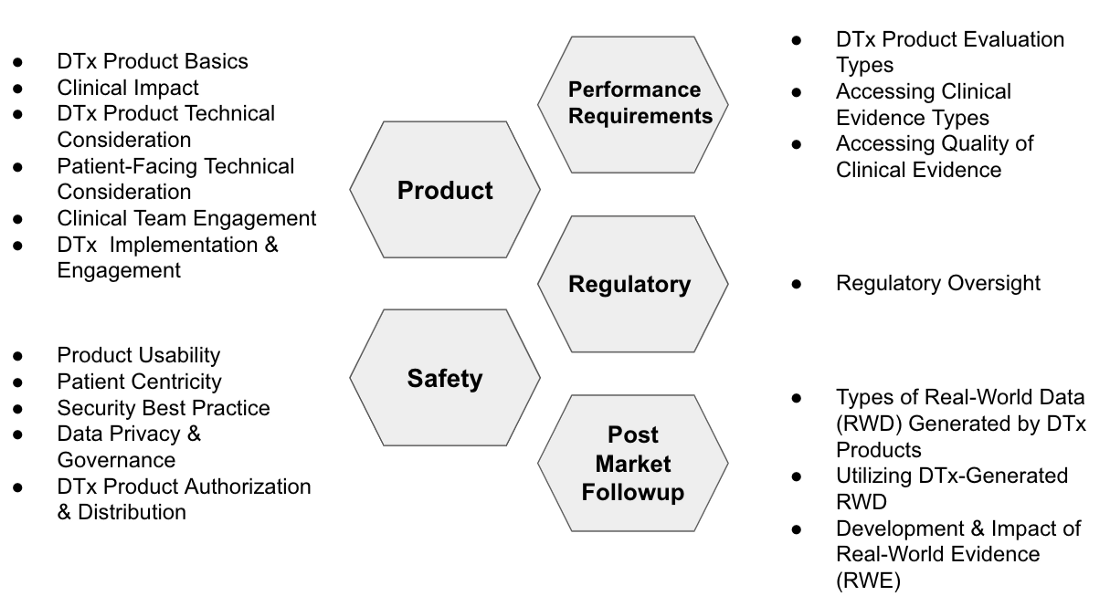
2. Six factors for product success
In addition to the above basic requirements for product launch, according to [Note 3], it proposes six key factors to ensure the success of DTx products: Interoperability, Socialization, Outcomes, and Engagement ), Intelligence, and Integration.
Interoperability , using advanced technologies to allow data sharing across multiple platforms.
Intelligence leverages advanced technologies such as machine learning, deep learning and artificial intelligence to produce personalized, evolving systems and interventions.
Socialization allows the exchange of cumulative or real-time patient data with other member affiliates.
Integration involves using DTx products as part of a patient's lifestyle, spending more time managing their own care and less time with clinicians.
In addition, outcomes (Outcomes), from preliminary studies to randomized controlled trials, are very important to building a successful DTx product.
Finally, gaming principals should be applied to DTx interventions to meet engagement .
From all these factors, interoperability should be the focus as it enhances big data processing, communication, qualified research and international cooperation [Note 2]
Therefore, it can be seen that compared with general medical materials or drugs, this type of products emphasizes the integration and interaction with users. Whether it is the use of artificial intelligence to respond or the assistance of medical care providers, it is foreseeable that services will account for Very important proportion.
In addition, if it is cross-border sales and encounters areas with different language systems, the performance of linguistic validation (Linguistic Validation), which has been receiving much attention recently in medical materials regulations, will be an important specification.
DTx product development role discussion
1. Ideal participating manufacturers
According to Deloitte's Crossing Boundaries - Exploring Digital Healthcare Regulatory Response Strategies [Note 4], the digital medical device ecosystem can be represented as shown in Fig2.
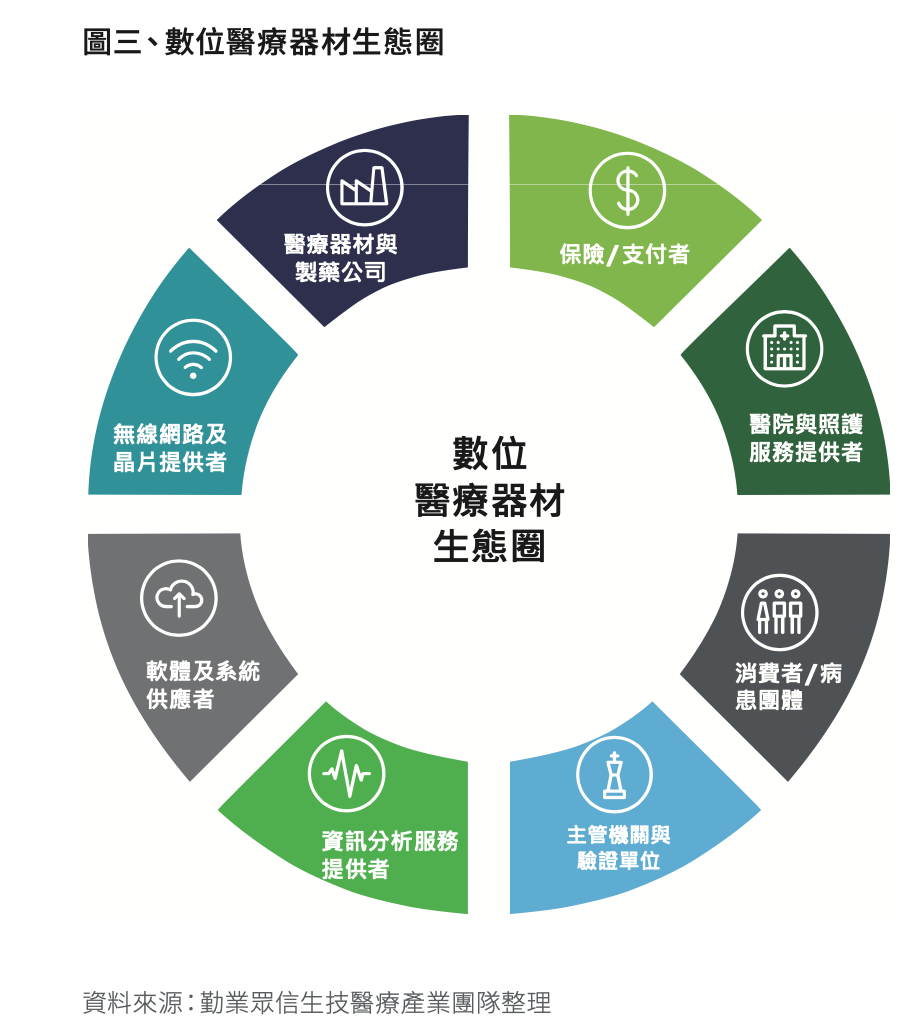
The ideal iron triangle of DTx developers is medical equipment, pharmaceutical companies, and information analysis service providers as shown in Figure 3.
From the perspective of marketing approval, the ideal combination of medical devices and pharmaceutical companies lies in the complementarity of regulations, because medical materials and drugs are governed by different laws, and the characteristic of DTx is that the relevant product regulations are mainly based on SaMD, that is, medical materials However, the methods of clinical trials are closer to those of pharmaceuticals.
As for the qualifications of a medical material manufacturer, its products need long-term third-party research and reference materials from other applied studies such as observational studies . Otherwise, a medical material without sufficient clinical research support cannot guarantee that the results of clinical trials will be Believable.
In terms of post-launch tracking and even customer service, information analysis service providers play the most critical role.
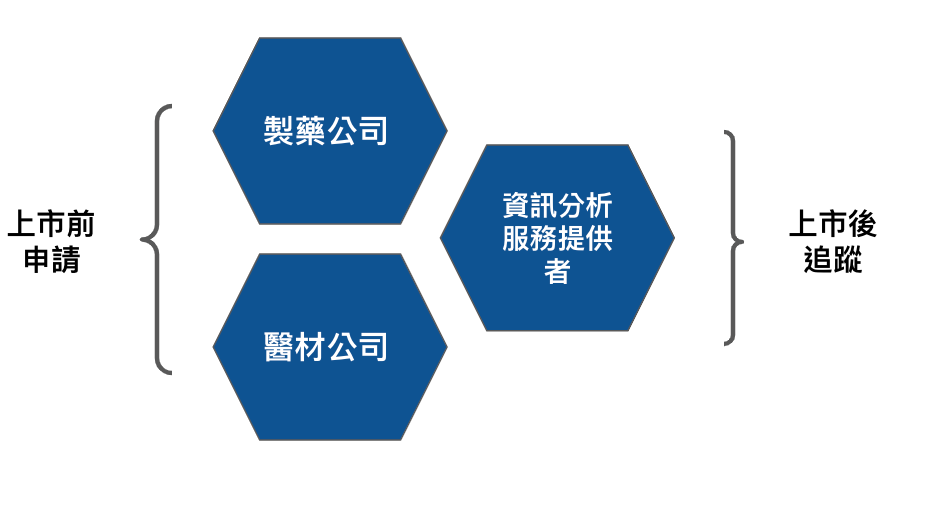
In addition, if it is behavioral therapy (CBT, Cognitive behavioral therapy), in addition to pharmaceutical companies matching medical equipment, the combination of medical centers and medical equipment companies is also another option to consider. This type of combination has another advantage in that it can be used on the server side. Directly leverage healthcare provider resources.
It can be seen that DTx is actually a product that requires the integration of different industries.
2. Organizational implementation
Because the basic requirement structure of regulations is similar to the US FDA’s Pre-Cert Program or AI/ML Framework, for discussions on related specifications, please refer to the following column
In addition to describing the relevant architecture, it also explains the requirements of relevant laws and regulations in the form of specifications.
As for the implementation of the organization, the author proposes the following designs for the relevant organizations based on the needs of the above regulations.
New generation medical software development system layout that complies with new FDA requirements
In addition, as far as the organization is concerned, because this is an organization that requires cross-industry integration, it is a task organization formed by medical equipment, pharmaceutical companies, and information analysis services. Because this is a professional field of medical regulations, the leader will be medical equipment or pharmaceutical companies.
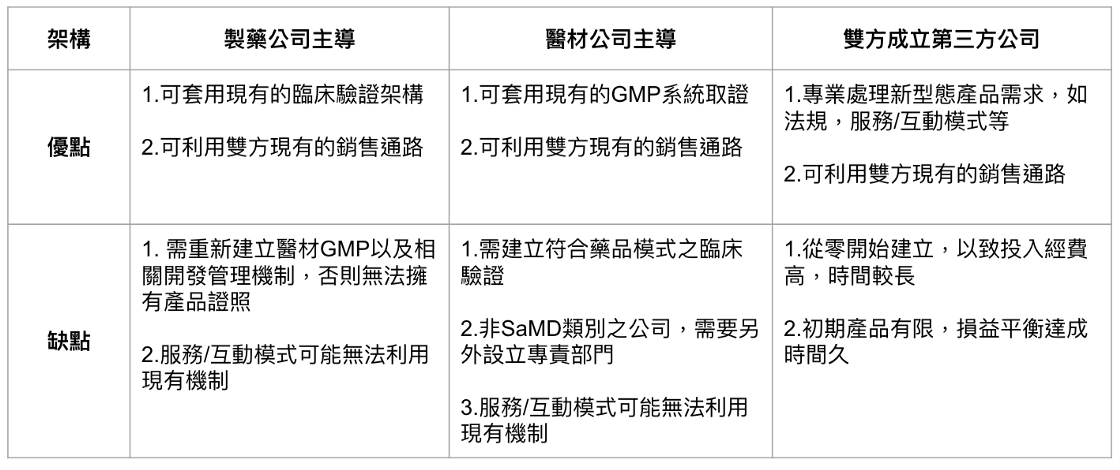
(1) Dominated by pharmaceutical companies, integrating medical material companies and information analysis service companies: The advantage is that digital therapy can be regarded as a type of medicine, so it can be used easily in clinical verification. Perhaps in some therapies, pharmaceutical companies are also the most Suitable for the production of digital therapy content.
However, the potential disadvantage is that if the pharmaceutical company has not established a separate GMP system for medical materials, in theory, the regulations need to be applied by the medical material company, because digital therapy is SaMD and software is a medical material category.
(2) Medical material companies take the lead and integrate pharmaceutical company information analysis service companies: The advantage is that they directly use the medical material company's GMP system to develop products, and the pharmaceutical company is the content provider.
However, the potential disadvantage is that in terms of clinical verification, the existing model of medical material companies may not be applicable, and it needs to rely on the assistance of pharmaceutical companies.
In addition, if the medical material company is not a SaMD-based company, it must set up an additional unit dedicated to SaMD in the organization. Although it can be included in the scope of the current Notify Body, it remains to be seen whether the extra business units are suitable for the current operations and whether they will cause a waste of resources. Excluded? This is an issue that needs to be considered.
(3) A pharmaceutical company and a medical material company set up a joint venture to establish a new company: the advantage is professional division of labor and does not affect the current organizational management, but the disadvantage is that additional resources need to be invested.
In addition, when a new company or business will bear the cross of profit and loss, it is also a consideration.
The three methods have their own advantages and disadvantages, as shown in Figure 4, which depend on the resources of both parties.
discuss
All in all, a company that wants to realize digital therapy products is an organization that combines the characteristics of a medical device, a pharmaceutical company, and an information analysis service.
Although it is a type of drug, GMP is the structure of medical materials. In addition, post-market tracking and in-depth user interaction are also important parts.
From the author's point of view, because this is a new type of product, the existing ecosystem does not have a completely suitable organization, so it is more reasonable to set up a dedicated company.
As for the challenge of profit and loss balance, in terms of practical operation, you may consider looking for medical material companies that mainly focus on SaMD products, or those whose main industry is software such as Teleheath and Remote Monitoring.
As far as this company is concerned, the resources required to add the DTx product are far lower than the re-establishment of a pharmaceutical company or the establishment of a new business by a hardware and medical device company, and even the operational efficiency is much higher.
Note 1: https://dtxalliance.org/advancing-dtx/dtx-value-guide/
Note 2: Accelerating Biopharmaceutical Innovation With Digital Therapeutics To Improve Patients' Quality Of Life
The Biofourmis Therapeutics Approach | Advancing Drug Development & Efficiencies
Note 3: Hong, JS, Wasden, C., & Han, DH (2021). Introduction of Digital Therapeutics. Computer Methods and Programs in Biomedicine, 106319
Note 4: Crossing Boundaries—Exploring Digital Healthcare Regulatory Response Strategies
Like my work? Don't forget to support and clap, let me know that you are with me on the road of creation. Keep this enthusiasm together!

- Author
- More