【法規工具文】Quick-guide on Software Pre-Cert Program (2) — Excellence Appraisal
【導讀】本文最初用英文寫作,目的是保留FDA原意。而本版的目的為工具用文章,故用中文註釋以便快速抓到重點,但細節部分還是建議細讀英文說明
個人這裡做一個貫穿的概述:
基本的卓越性評鑑架構請參考Fig 1,在這裏FDA告訴廠商其評鑑的範圍(domain)及元件(Elements)是什麼?下一步就是廠商根據FDA提出的要求及格式,呈現證據證明該公司如何符合CQOE的原則。
最後FDA評鑑後,決定廠商是Level 1還是Level 2。這個Level 1或是Level 2會決定了下一關,審核路徑的途徑的複雜程度。
Organizations that produce safer and more effective device software products not only “do the right things,” but they also “do the things right” based on evidence that informs better decision- making.
Excellence Appraisal , the 1st component of Pre-Cert program, is the evaluation upon how the organization “do the things right”.
Fig 1 is the conceptual framework and serves the basis of this chapter.
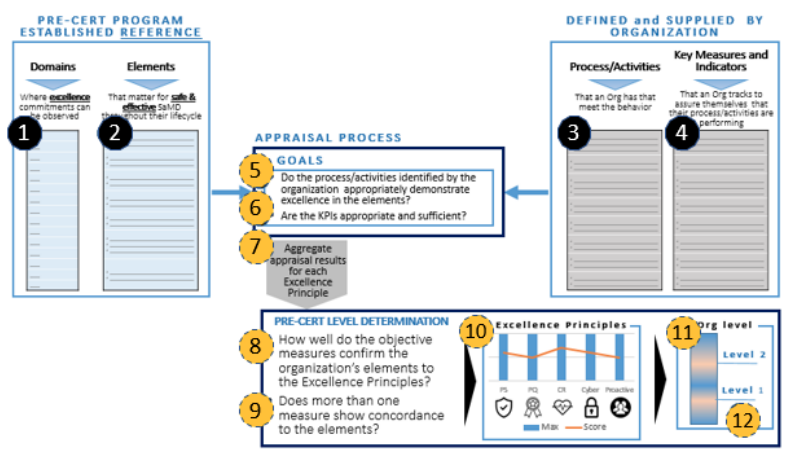
Logics behind the 1st Component
According to Fig 1, (1) Pre-Cert Program established reference is proposed as appraisal elements, and then (2) the organization should supply the process/activities, and key measures & indicator with respect to the elements.
(3) Based on the supplied information, after appraisal process, Pre-Cert level will be determined. The level will influence the review pathway, which is the 2nd component.
【導讀】這裡先從FDA要求如範圍,元件部分等逐一介紹,Table 1 則是FDA評鑑卓越性的例子,可視為FDA的規格書
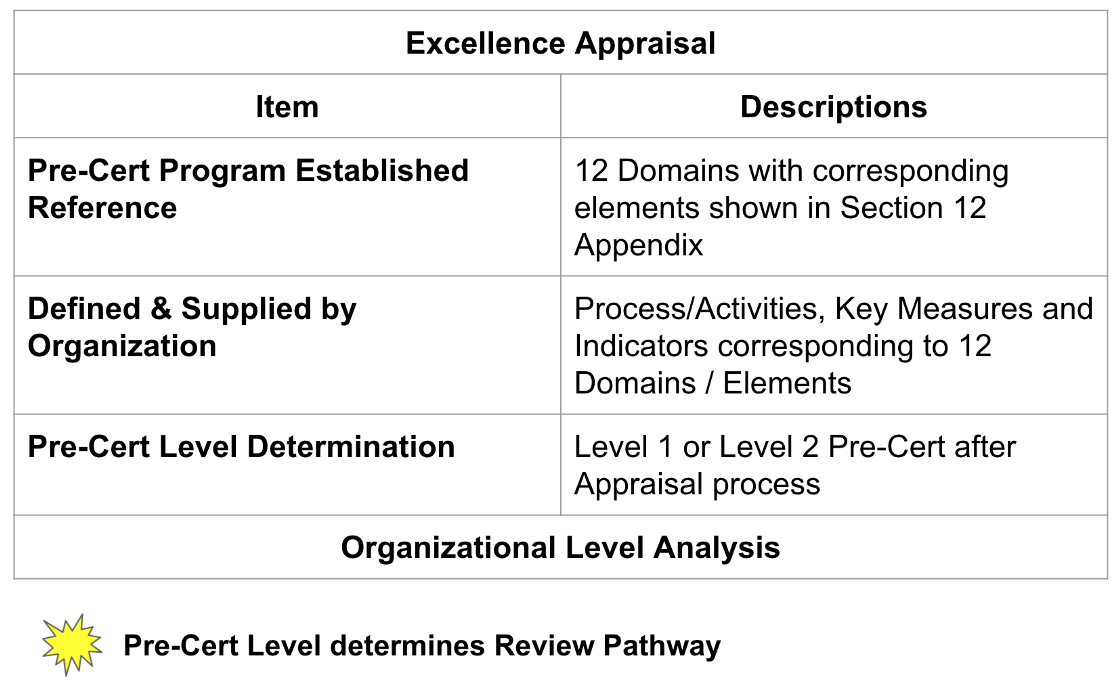
Pre-Cert Program Established Reference
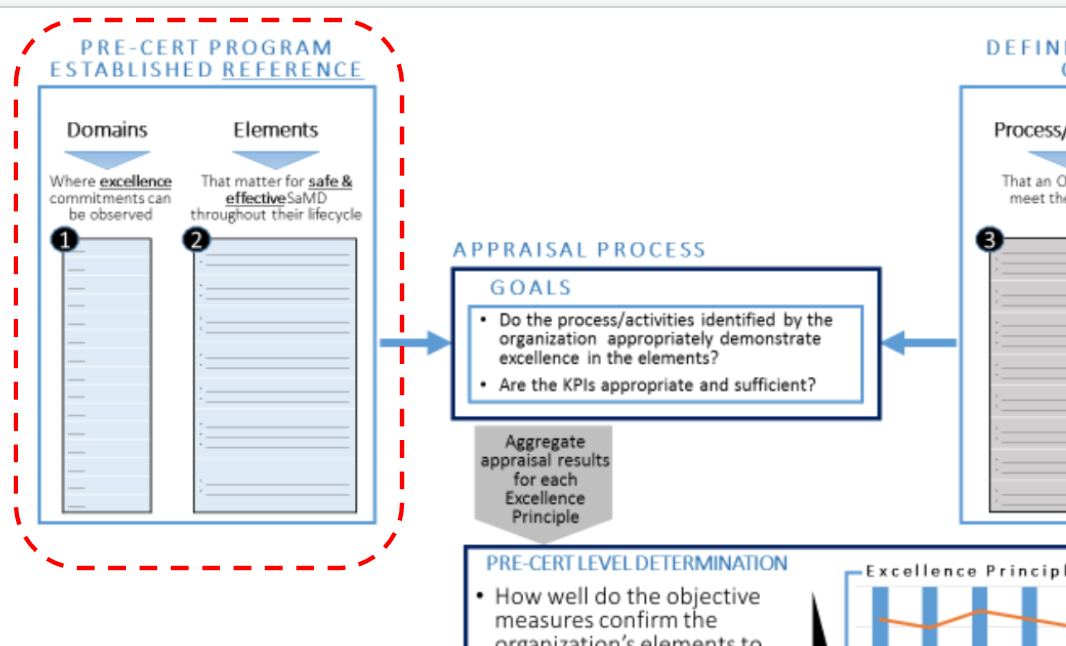
Pre-Cert Program established reference (Fig 2)consisted of domains and Elements.
Domain: Where excellence commitment can be observed.
Elements: The matter for safe & effective SaMD through their lifecycle.
The example of the appraisal can be found in Table 1. One organization domain may be with several elements, and each element will be evaluated with Excellence Principles (aka CQOE principles).
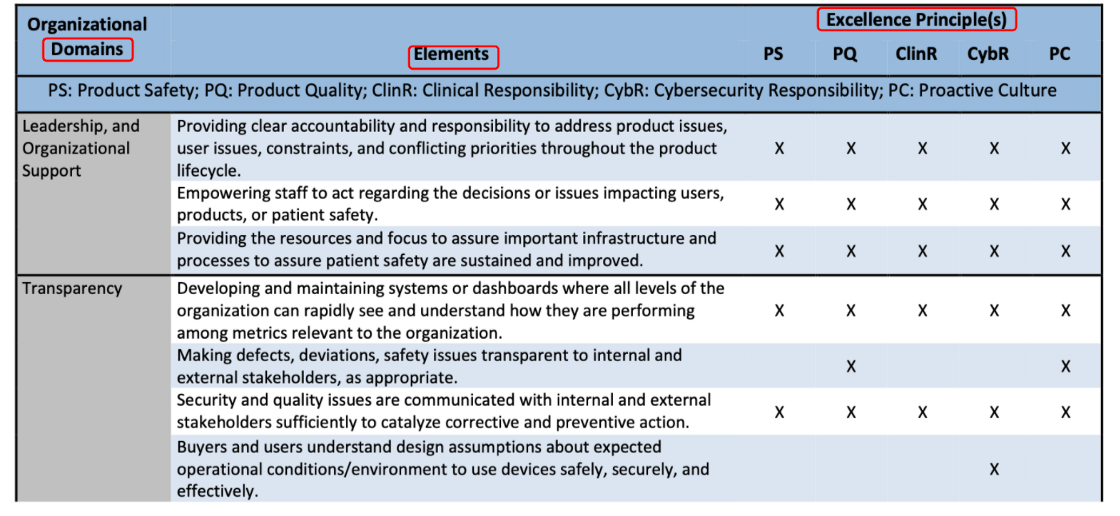
【導讀】以下有12項FDA評鑑的範圍(Domain)
A full list of elements and domains, mapped to Excellence Principles the organizations support, can be found in Section 12 Appendix of “The Software Precertification Pilot Program version 1.0 Working Model”.
- Leadership and Organizational Support — Elements related to the organization’s leadership establishing the strategic direction, responsibility, authority, and communication to assure the safe and effective performance of the SaMD.
- Transparency — Elements related to the organization’s open sharing of relevant information with all stakeholders to build confidence in the organization and its products.
- People — Elements related to providing appropriate resources as needed for ensuring the effectiveness across all lifecycle processes and activities in meeting user requirements.
- Infrastructure and Work Environment — Elements related to the availability of infrastructure such as equipment, information, communication networks, tools, and the physical facility throughout SaMD lifecycle processes.
- Risk Management: A Patient Safety Focus — Elements related to monitoring and managing risks along multiple dimensions such as user-based, application-based, device-based, use environment-based, and security-based across all lifecycle processes.
- Configuration Management and Change Control — Elements related to identifying and defining the software configuration and controlling the release and change of the software throughout all lifecycle processes.
- Measurement, Analysis, and Continuous Improvement of Processes and Products– Elements related to managing and improving product realization and use through real- world performance monitoring.
- Managing Outsourced Processes, Activities, and Products — Elements related to understanding, maintaining control, and managing the effect of outsourced activities, processes or products.
- Requirements Management — Elements related to clear, and often repeated user interaction to understand and clearly articulate user needs throughout all lifecycle processes.
- Design and Development — Elements related to ensuring safe, effective, and secure SaMD based on user and other performance requirements during all lifecycle phases at key milestones and good development practices incorporating appropriate review activities such as code review, peer review, and self-review.
- Verification and Validation — Elements related to understanding the criticality and impact to patient safety by providing assurance of conformity to requirements and reasonable confidence that the software meets its intended use/user needs and operational requirements.
- Deployment and Maintenance — Elements related to activities such as delivery, installation, setup, and configuration of software including documentation and user training materials that identify any limitation of the algorithm, provenance of data used, assumptions made, etc. that should be considered during deployment. Additionally, modification of previously deployed software while preserving the integrity of the software by not introducing new safety, effectiveness, performance, and security hazards.
The above mentioned can be considered as the requirements.
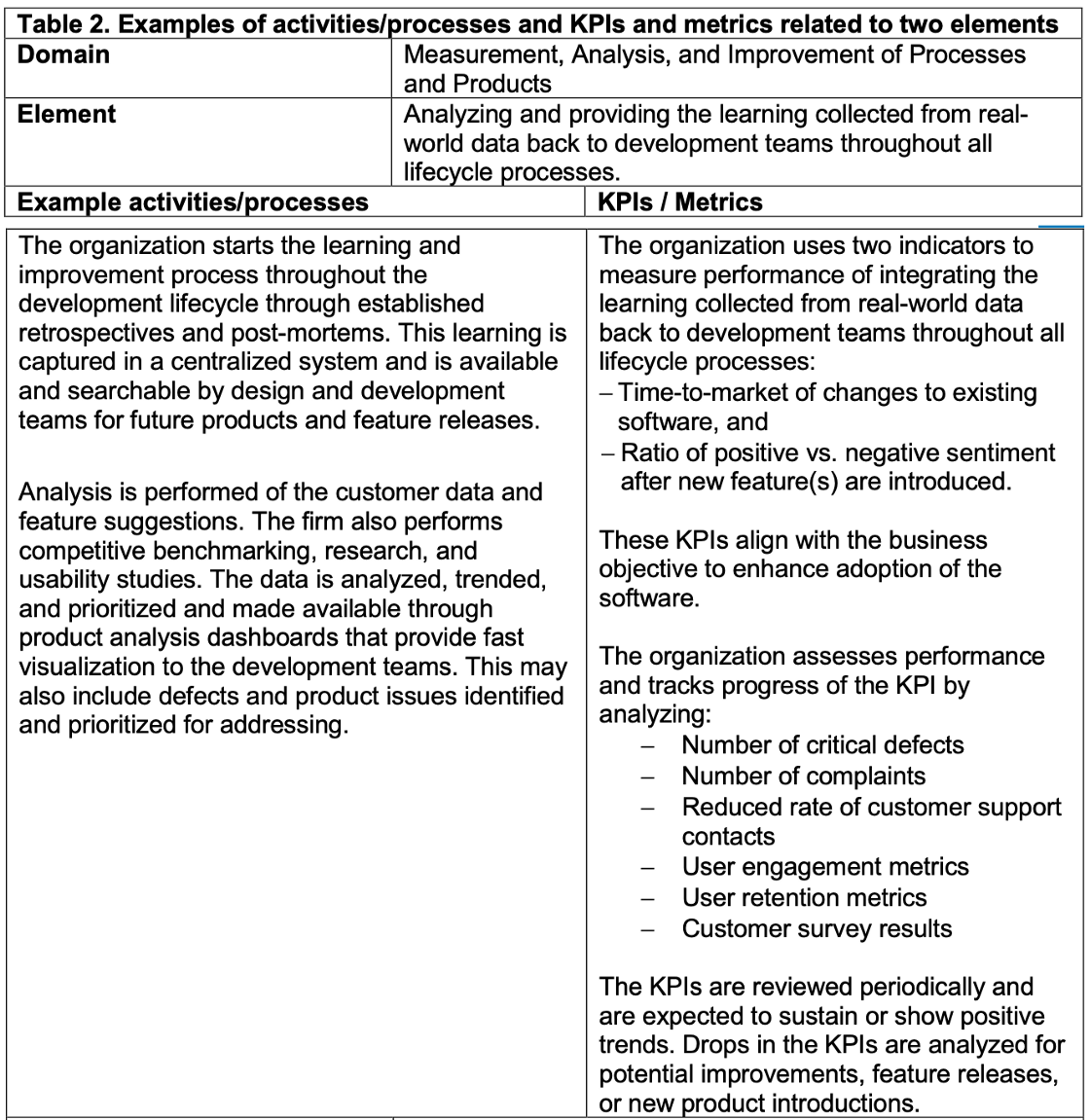
【導讀】以下則是建議廠商就FDA的要求,所需要回覆的格式, Table 2
Key Performance Indicators & Appraisal Process
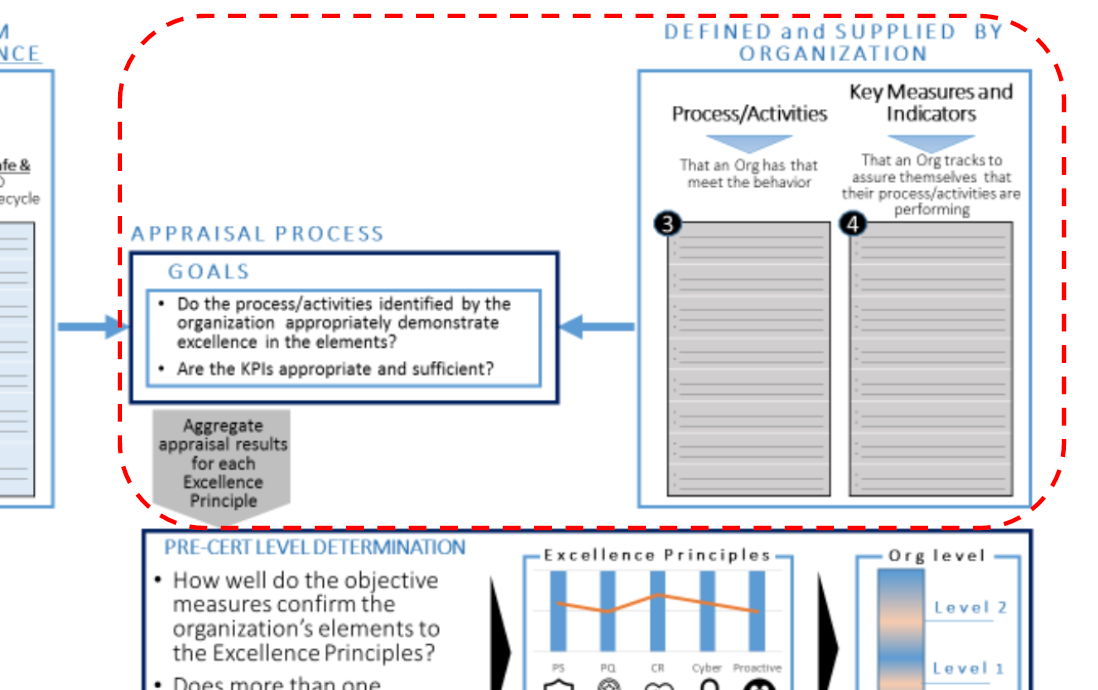
Although the appraisal method is not fully developed, the FDA generally intend to evaluate organizational elements based on objective, observable evidence. Each organization would determine which processes/activities and Key Performance Indicators (KPIs) best meet these elements for purposes of meeting regulatory requirements.
Example of activities/processes and KPIs related the element of the Excellence Appraisal are shown as following.
By aggregating and analyzing collected information, the FDA can understand how organizations build safe and effective SaMD, how they know the devices are safe and effective in the real world, and how they improve safety and effectiveness, as well as efficiency and time to market.
【導讀】卓越性評鑑也認同ISO的標準,如13485,14971,62304等
The Excellence Appraisal incorporates the following development principles:
- Designed for organizations of all sizes
- Allows organizations to demonstrate excellence based on outcomes achieved by their unique processes, operations, and capabilities
- Applies least burdensome approach by observing organizations’ current processes
- Recognizes organizations following existing standards (e.g., Quality System Regulations, ISO 13485, ISO 12207, ISO 62304, ISO 14971, ISO 9001) and outcomes achieved by following those processes
【導讀】最後進入評鑑,決定是那個Level
Precertification Level Determination
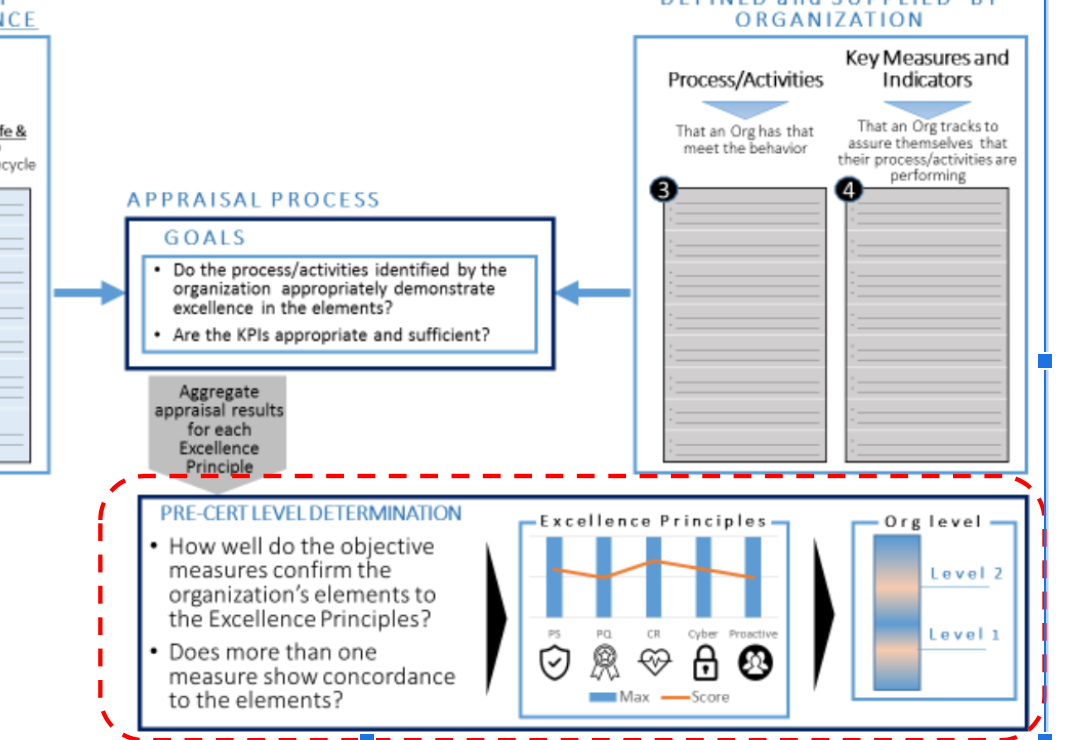
The goal of establishing levels of precertification is to maintain the same standards of safety and effectiveness of products marketed today for software manufactured by precertified companies. The levels of precertification are intended to provide, to both FDA and the users, confidence in an organization’s ability in developing, maintaining, and marketing safe and effective SaMD.
The FDA has proposed two levels of precertification based on an organization’s excellence. FDA expects that the Excellence Appraisal would be able to identify the performance of various types of organizational structures and normalize that performance using the Excellence Principles( aka COQE principles), illustrated as Fig 5.
【導讀】這根據各個範圍(Domian)評鑑的結果,加總得出成績
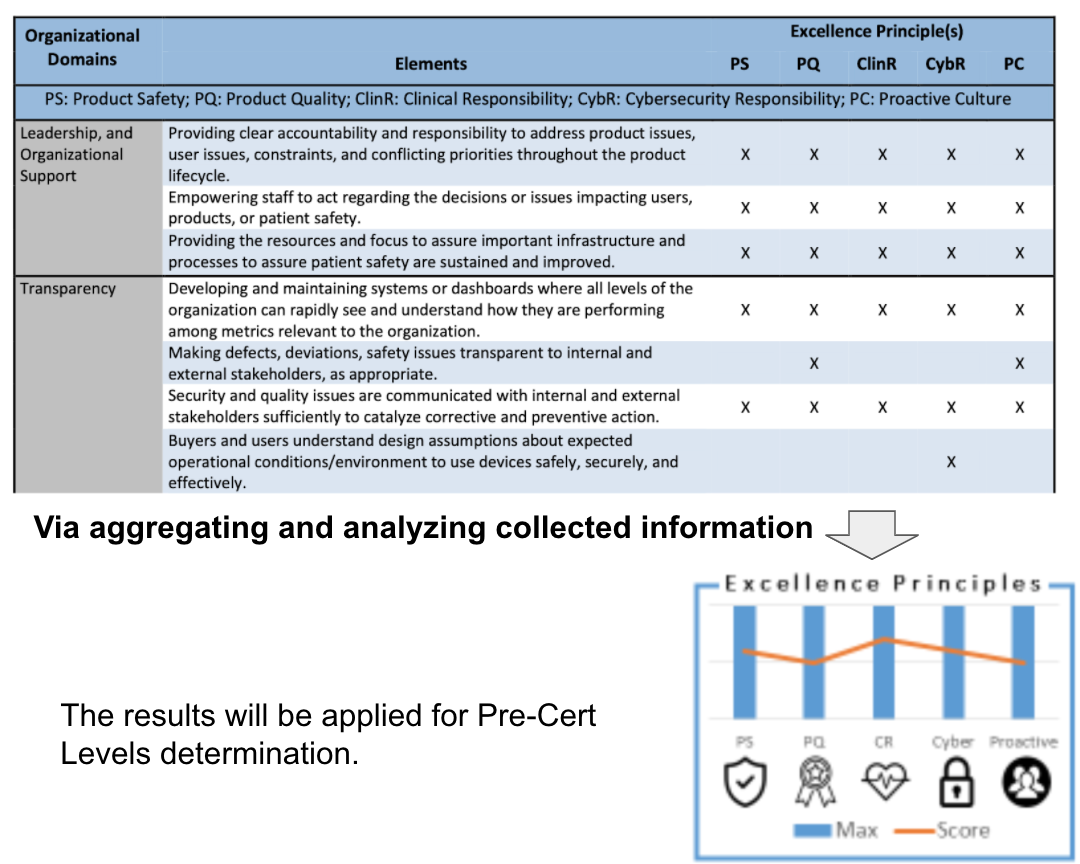
【導讀】最後決定等級
Level 1 Pre-Cert — This level of certification is designed to allow organizations to develop and market certain lower risk software without review while requiring a streamlined review for other types of software. The FDA envisions this level would be awarded to an organization that has objectively demonstrated excellence in product development in all five Excellence Principles, with a limited track record in developing, delivering and maintaining products.
Level 2 Pre-Cert — This level of certification is designed to allow organizations to develop and market certain lower and moderate risk software without review while requiring a streamlined review for other types of software. The FDA envisions this level would be awarded to an organization that has objectively demonstrated excellence in product development in all five Excellence Principles, with a proven track record in developing, delivering and maintaining products.
Specific types of SaMD that would require streamlined review or not for the two Pre-Cert levels are described in the Component 2, Review Pathway Determination.
【導讀】以下為一個總結說明卓越性評估的階段,及其產出
Summary
Like my work? Don't forget to support and clap, let me know that you are with me on the road of creation. Keep this enthusiasm together!