Playing Electric Every Day Can Cure ADHD - The First FDA Cleared Mission Game EndeavorRX
Digital medical treatment has been discussed for a long time. When it is really used in medical treatment, certification is necessary, only doctors can use it, and insurance can only be activated. EndeavorRX, the first-ever approved digital medical game, is a prescription-only game said to improve attention function in children with attention deficit hyperactivity disorder (ADHD).
The U.S. Food and Drug Administration (FDA) approved a video game to treat diseases in June .
Developed by Akili Interactive Labs, the product is the first FDA-approved game-based treatment for the disease and the first digital treatment approved for ADHD, according to the agency.
Setting a regulatory precedent in the field of digital therapeutics has given other digital health companies a boost and confidence in this nascent industry. "It means the FDA can regulate these interventions," said Jo Masterson, co-founder and CEO of 2Morrow , which develops digital therapeutics to help people quit smoking, lose weight, release stress, and make behavioral changes that improve health.
Digital therapy is a software-based disease treatment device.
For example, therapy may be in the form of messages or visual stimuli and delivered via telephone, computer or other digital device. Companies that want to claim that soft medical materials treat or diagnose diseases often must obtain FDA clearance before selling them to consumers.
And FDA-cleared digital medical products must be separated from the plethora of health-promoting software on the market.
Headspace has developed a meditation app that can help people "reduce stress" and "improve their mood." Because this health claim does not involve treating a disease , Headspace can be sold directly to the public without FDA review.
Still, some digital therapeutics companies believe that being an FDA-approved treatment will eventually pay off. A handful of players in the digital space have successfully passed the U.S. regulatory review process.
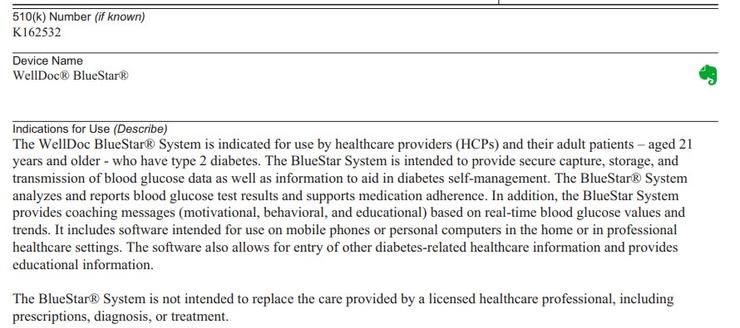
WellDoc, an FDA-approved prescription-only app called BlueStar in 2014, acts as a digital assistant for people with type 2 diabetes, helping them determine when to test their blood sugar and how to manage it with medication, food, and exercise. blood sugar.
Pear Therapeutics, which received FDA approval in 2017, launched a prescription-only app that offers a digital form of cognitive behavioral therapy that can help people with alcohol, marijuana, cocaine and stimulant substance use disorders maintain their cravings. The company has since licensed marketplace apps that provide information and counseling to support people with opioid use disorder and chronic insomnia.
Akili's new software, EndeavorRx, offers therapy not as information or counseling, but as an engaging video game. By presenting both sensory and motor stimuli, the game teaches children with ADHD to manage competing cognitive tasks, shift attention between tasks, and ignore distractions. Eddie Martucci, Akili's chief executive, said the software is priced roughly in the range of traditional ADHD medications.
Akili decided from the beginning to make FDA approval part of its business model. "When we started the company, we invested early. So in that sense, we were very much like a biotech company," Martucci said. "Our long-term model is that the digital therapeutics industry should have products that are like drugs in almost every way except interaction. That means doctors have the right to prescribe and insurance companies have the right to cover it, just like a drug."
Only time will tell if this business model works. "We won't know for a few quarters," Martucci said. Part of that depends on how doctors integrate the game into their care, and whether payers will pay for it, he said. But Martucci said he believed it was worth taking the regulatory route. "Nothing moved me that it was a good decision," he said. Akili has raised $120 million so far.
In filing its lawsuit with the FDA, Akili provided data from five clinical trials, all of which examined safety and efficacy . Having clinical efficacy data "is very important in this new FDA process," Martucci said. "The vast majority of questions and conversations with the FDA are about clinical benefit to patients, " Martucci said.
Akili claims its products improve cognitive function. The cornerstone of the company's evidence comes from a randomized controlled trial involving 348 children with ADHD, published in the journal The Lancet Digital Health in February .
About half of the participants in the study played Akili's game, while the other half played a fake game created for research purposes. Both groups played 25 minutes a day, 5 days a week, for nearly a month.
The trial researchers measured improvements in attention based on the children's scores on a computerized test of attentional performance known as the Test of Variables of Attention (TOVA) Attention Performance Index (API), which was administered to the children before and after the intervention period. Children who played EndeavorRx improved their TOVA API test scores than those who played the dummy game -- 0.93 in the experimental group and 0.03 in the control group. Of the kids who played EndeavorRx, 36 percent no longer exhibited attention deficits on at least one measure of attention, compared with 21 percent of the control group.
Whether this translates into improvements in real-life attentional function is unclear. "For pretty much anything, you can show that doing something more and more makes you better at that thing, but how much does this transfer to a real-world situation?" Xealth, a digital platform company David Cooper, a psychologist and manager of strategic partnerships, said the company combines digital health tools with electronic health records. "The real question to ask is: Are these kids actually getting better at school? Are they better able to focus and better regulate their emotions?"
Martucci said he agrees that products need to show benefit beyond treatment, which is why his company's pivotal trial measures efficacy based on improvements to the TOVA API -- a long and boring computer-based test -- rather than on Improvements to the game itself. A follow-up study using parent-reported assessments as an endpoint is underway.
Other discussions with the FDA have focused on other novel elements of Akili's game and trial design, Martucci said. For example, video games adapt in real time to a child's abilities and progress. So unlike traditional medicine where every pill is the same, Akili's video game therapy varies slightly from one "dose" to the next and from patient to patient.
Despite the regulatory success of Akili, Pear and others, some decision makers in the digital therapeutics space see no need to all seek FDA approval.
For example, makers of smoking cessation apps are building up a lot of evidence through clinical trials and finding markets through healthcare providers without the FDA's seal of approval, 2Morrow's Masterson said. “We generally know that health insurance plans want to pay for FDA-approved interventions — and that’s a valid point. However, we now get paid by health plans through their health and wellness plans instead of moving in that direction. ,"she says. The prospect of engaging with the FDA is still on her company's table, Masterson said, but was not initially part of the business plan.
Xealth 's Cooper added, "If you're a company, the first thing you want to do is reduce operational risk. There's a lot of uncertainty and risk in the digital health space right now. FDA audits are beneficial and helpful, but the whole field is new, It's constantly changing and still has uncertainty," he said.

COVID-19 has exacerbated this uncertainty. When the pandemic hit earlier this year, the FDA made a notable change to digital health policy: As an emergency measure, it would allow digital therapeutics to enter the market without regulatory oversight. The change is part of an effort to provide people with mental health care during quarantines and economic shutdowns, and applies to a variety of therapies that do not pose undue risks when treating certain mental illnesses, including obsessive-compulsive disorder, insomnia, depression , substance use disorder, autism and ADHD. This policy remains in effect during an emergency.
When the FDA announced the deregulation , Akili was awaiting the FDA's decision on EndeavorRx. That was in April, and the company brought the EndeavorRx to market within a week. "There were a lot of sleepless nights that week," Martucci said. Since then, at least two companies have taken advantage of the loosening of the rules.
Pear released its schizophrenia product candidate Pear-004 to the public in a limited manner, which has not yet been approved by the FDA. Uppsala, Sweden-based Orexo announced in July that it would launch two treatments in the U.S. — one for depression and one for problematic alcohol use — and accelerate its software for opioid use disorder. test. These therapies are based on cognitive behavioral therapy—a type of digital counseling.
The loosening of the rules gives companies the opportunity to collect data on the efficacy of their software in the real world.
"This is a unique opportunity to do this and make products available as they go," said Dennis Urbaniak, executive vice president of digital therapeutics at Orexo . He said his company still plans to seek FDA approval for its products it releases to the public. approve.
It's unclear how or if the agency will roll back the policy after the pandemic is over. Companies do not need to inform the FDA that they are taking advantage of the relaxed rules.
The FDA does come up with a set of recommended criteria that companies should meet when releasing digital therapeutics to the public. "Anyone moving forward under these pathways needs to be prepared, whether they formally notify the FDA or not, the FDA is expected at some point to look at what they are doing and whether they meet these standards,"
REF:
https://www.nature.com/articles/s41587-020-0726-6
Like my work? Don't forget to support and clap, let me know that you are with me on the road of creation. Keep this enthusiasm together!

- Author
- More