
葉峻榳醫師,新陳代謝暨內分泌專科,專攻糖尿病、肥胖等疾病。熱愛運動,朝著整合「醫學」與「運動」的目標,幫助人們變得更強壯。 葉峻榳醫師:https://chunting.me/ 葉峻榳醫師診療室:https://www.yehclinic.com/blog
《Obesity》📖A complete guide to legal weight loss drugs✨
Article source: https://www.yehclinic.com/list-of-weight-loss-medications/
According to statistics from the National Health Administration of the Ministry of Health and Welfare, the prevalence of overweight and obesity among Taiwanese adults is 43%, with a male rate of 48.9% and a female rate of 38.3%; 1 out of 3 want to lose weight. According to the World Obesity Federation (2015), Taiwan is the champion in Asia in terms of adult and child obesity rates!
Obesity treatment, diet adjustment and exercise intervention, and drug therapy also play an important role. There are all kinds of health food related to weight loss on the market, and there are also medical institutions that provide so-called "cocktail therapy". So, what are the weight loss drugs? Let's find out 👀
📖Number of characters: 3219 characters.
Banned weight loss drugs
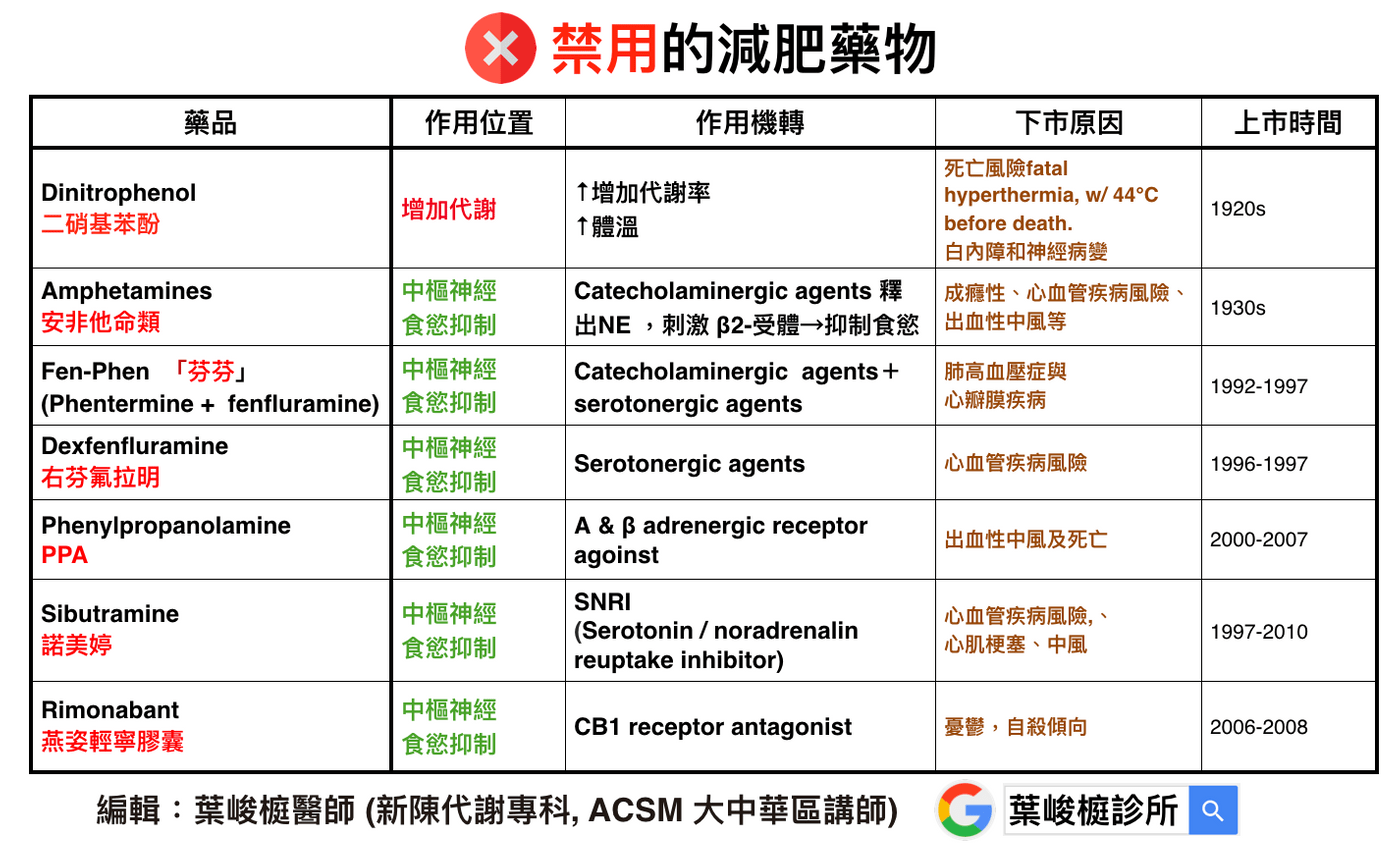
Over the past few decades, a number of drugs have been published to address obesity; several of these drugs have been removed from the market because of adverse effects, including cardiovascular problems. An example is as follows:
✖️Dinitrophenol (dinitrophenol)
1⃣️Mechanism of action: ↑Increase metabolic rate, ↑Body temperature.
2⃣️ Reasons for delisting: risk of death (fatal hyperthermia, w/ 44°C before death), cataract and neuropathy, etc.
3⃣️ Listing time: 1920s.
✖️Amphetamines
1⃣️Mechanism of action: Catecholaminergic agents release NE, stimulate β2-receptors → suppress appetite.
2⃣️ Reasons for delisting: addiction, risk of cardiovascular disease, hemorrhagic stroke, etc.
3⃣️ Time to market: 1930s.
✖️Fen-Phen Phentermine + fenfluramine
1⃣️Mechanism of action: Catecholaminergic agents+serotonergic agents → suppress appetite.
2⃣️ Reasons for delisting: pulmonary hypertension, heart valve disease.
3⃣️ Time to market: 1992-1997.
✖️Dexfenfluramine
1⃣️Mechanism of action: Serotonergic agents → suppress appetite.
2⃣️ Reason for delisting: cardiovascular disease risk.
3⃣️ Time to market: 1996-1997.
✖️Phenylpropanolamine (PPA)
1⃣️Mechanism of action: Alpha & β adrenergic receptor agoinst → suppress appetite.
2⃣️Reason for delisting: hemorrhagic stroke, death.
3⃣️ Time to market: 2000-2007.
✖️Sibutramine
1⃣️Mechanism of action: Serotonin / noradrenalin reuptake inhibitor (SNRI) → suppress appetite.
2⃣️ Reasons for delisting: cardiovascular disease risk, myocardial infarction, stroke.
3⃣️ Time to market: 1997-2010.
✖️Rimonabant Yanzi Qingning Capsules
1⃣️Mechanism of action: CB1 receptor antagonist → suppress appetite.
2⃣️ Reasons for delisting: depression, suicidal tendencies.
3⃣️ Time to market: 2006-2008.
FDA Approved Weight Loss Drugs
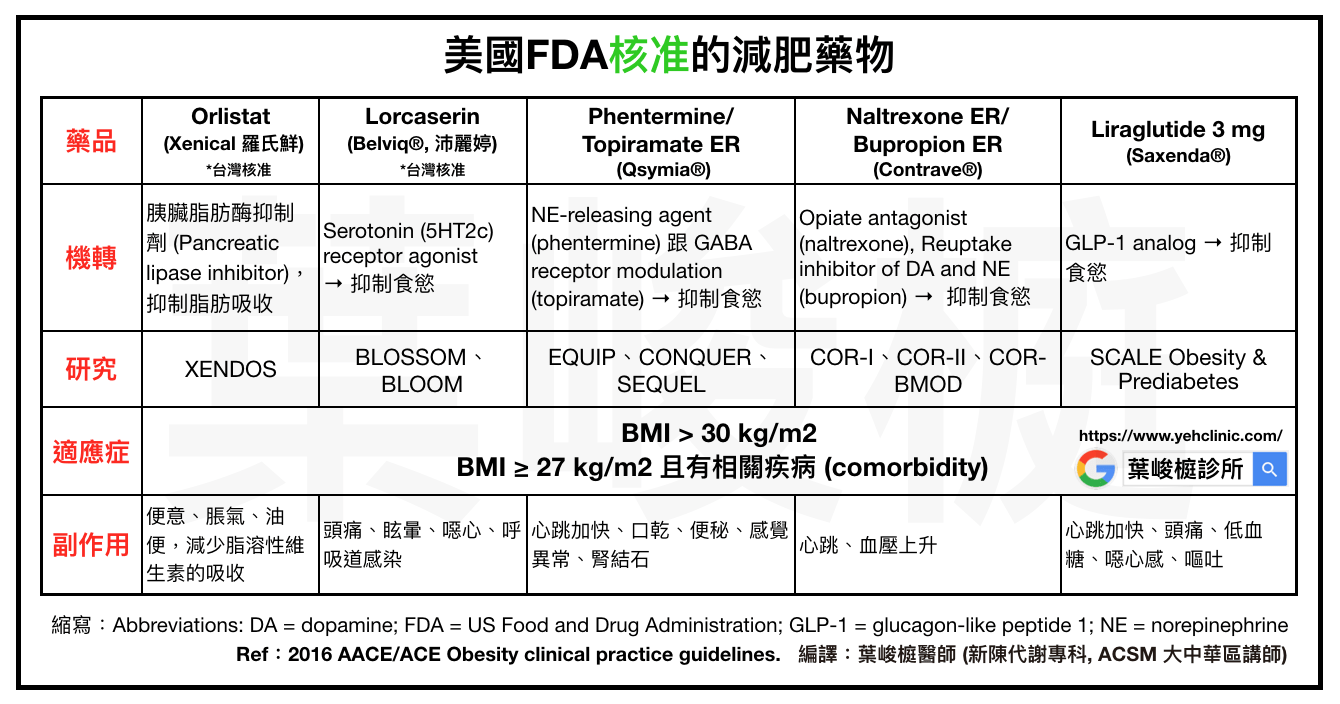
Currently, the "legal" weight loss drugs approved by the US FDA are as follows:
☑️Orlistat (Xenical, Roche Fresh)
☑️Lorcaserin (Belviq®, Pelletine)
☑️Phentermine/Topiramate ER (Qsymia®)
☑️Naltrexone ER/ Bupropion ER (Contrave®)
☑️ Liraglutide 3 mg (Saxenda®)
✨According to the regulations of the US FDA, the above weight loss drugs can only be used under the following conditions:
⭕️BMI > 30 kg/m2
⭕️BMI ≥ 27 kg/m2 and related diseases (comorbidity)
☑️Orlistat (Xenical®, Roche Fresh)
1⃣️Location of action: Gastrointestinal tract.
2⃣️Mechanism of action: Pancreatic lipase inhibitor, inhibits fat absorption.
3⃣️Study: XENDOS → Compared with placebo, 4.0% more body weight can be lost in 1 year and 2.6% more body weight can be lost in 4 years.
4⃣️Side effects: Unbearable constipation, flatulence, and oily stools, reducing the absorption of fat-soluble vitamins (vitamins A, D, E, K).
(Study Duration: % TBWL Greater Than Placebo. % TBWL = percent total body weight loss from baseline over that observed in the placebo group)
☑️Lorcaserin (Belviq®, Pelletine), launched in 2012
1⃣️Location of action: Central nervous system.
2⃣️Mechanism of action: Serotonin (5HT2c) receptor agonist → suppress appetite.
3⃣️Research: BLOSSOM and BLOOM study 🔜 Compared with placebo, 3.0%-3.6% more body weight can be lost in 1 year, and 3.1% more body weight can be lost in 2 years. After that, BLOOM-DM, CAMELLIA-TIMI 61 and other studies were published.
4⃣️Side effects: headache, dizziness, nausea, respiratory tract infection, etc.
☑️Phentermine/Topiramate ER (Qsymia®), launched in 2012
1⃣️The mechanism of action: NE-releasing agent (phentermine) and GABA receptor modulation (topiramate) → suppress appetite.
2⃣️Studies: EQUIP, CONQUER, SEQUEL studies → Compared with placebo, 8.6%-9.3% more body weight (high dose) and 6.6% body weight (general therapeutic dose) can be lost in 1 year. 2 years can lose 8.7% more body weight (high dose), 7.5% body weight (general therapeutic dose).
3⃣️Side effects: rapid heartbeat, dry mouth, constipation, paresthesia, kidney stones, etc.
☑️Naltrexone ER/ Bupropion ER (Contrave®), launched in 2014
1⃣️Mechanism of action: Opiate antagonist (naltrexone), Reuptake inhibitor of Dopamine and Norepinephrine (bupropion) → suppress appetite.
2⃣️Studies: COR-I, COR-II, COR-BMOD → Compared with placebo, 4.2%-5.2% more body weight can be lost in 1 year.
3⃣️Side effects: heartbeat, blood pressure rise, etc.
☑️Liraglutide 3 mg (Saxenda®), launched in 2014
Liraglutide (0.6mg, 1.2mg, 1.8mg) is regulated as a diabetes drug in Taiwan. In addition to lowering blood sugar, it also has the effect of weight control. At present, the high-dose Liraglutide 3 mg (Saxenda®) has not been listed in Taiwan. It is already available in South Korea, commonly known as " Slimming Needle ".
1⃣️Mechanism of action: GLP-1 analog → suppress appetite.
2⃣️Study: SCALE Obesity & Prediabetes → 5.6% more weight loss in 1 year compared to placebo.
3⃣️Side effects: rapid heartbeat, headache, hypoglycemia, nausea, vomiting, etc.
Taiwan Approved Weight Loss Drugs

Currently, there are only two "legal and compliant" weight loss drugs approved in Taiwan: Orlistat (Xenical) and Lorcaserin (Belviq®, Pelitine) . In other words, if you are currently taking weight loss drugs, and you take more than two kinds of drugs, the excess must contain drugs that belong to Off-Label Use (off-label use).
Therefore, if the weight loss drugs you are taking are not Orlistat (Xenical®, Roche Fresh) or Lorcaserin (Belviq®, Pelitine), theoretically, they cannot be advertised as weight loss drugs in Taiwan. So, will eating it hurt the body? Then it depends on what kind of medicine you take in...
summary
In the face of obesity, "diet, exercise, drugs and metabolic surgery" are all part of the weight management puzzle. Medicines, if used well and correctly, will become a weapon against obesity; if the wrong medicines are used, it may harm the body and the gains will outweigh the losses.
Author: Dr. Ye Junju (Metabolism Specialist, Lecturer in Greater China of American Sports Medicine Association , Medical Consultant of Ogma Fitness Center , Consultant of Compal Computer Project )
References
📖 2016 AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY COMPREHENSIVE CLINICAL PRACTICE GUIDELINES FOR MEDICAL CARE OF PATIENTS WITH OBESITY. Endocr Pract. 2016 Jul;22 Suppl 3:1-203. .
Like my work?
Don't forget to support or like, so I know you are with me..
Comment…