
服務生醫產業超過25年,經歷研發/產品管理/事業開發/銷售業務/品保法規等工作,工作橫跨美國,台灣,產品經歷家用醫材/專業醫材/實驗室設備等,在這個園地貢獻自己一點經驗及想法。
Discussion on the layout of digital therapy (3) - product realization
Discussion on DTx Product Specifications
Product specifications can be discussed from the DTx-Value-Assessment-Guide. Although the purpose of this guideline is to provide a reference criterion for evaluating the value of DTx in the health care delivery system, these value evaluation references actually express how the specifications of the product should be expressed. Open and plan.
1. Specifications
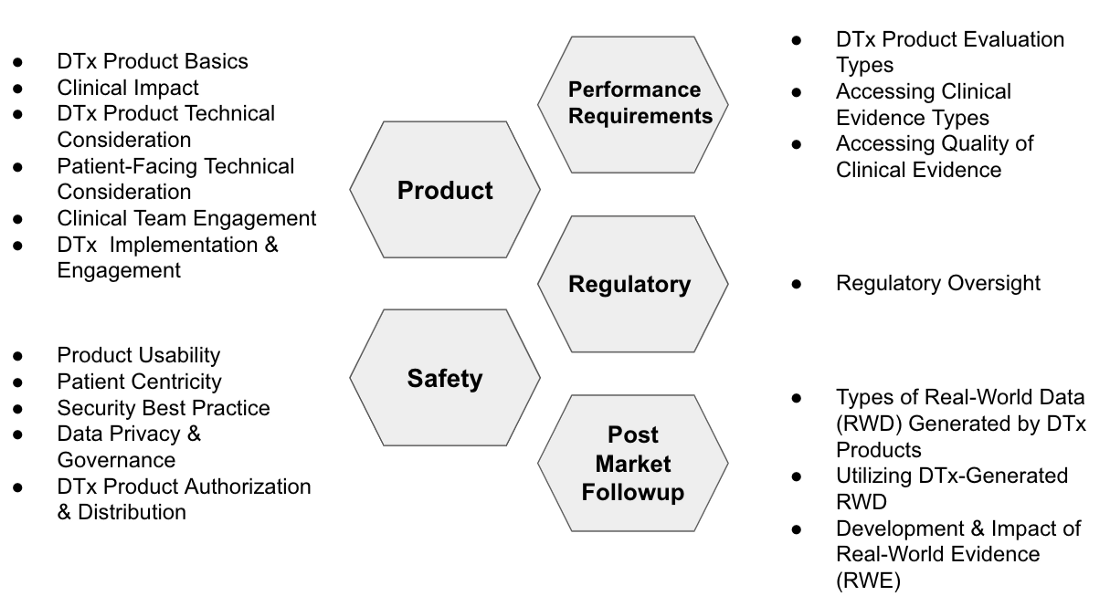
Using the general principles of medical material product development requirements, the products are classified from five principles: product requirements, functional compliance, safety compliance, regulatory approval, and post-market tracking.
Why use Post market follow up instead of Post Market Surveillance? The main reason is that post market surveillance is the general direction that the quality system must implement. For products such as DTx, after the quality system is listed Under the supervision structure, the most important work is the post-market tracking.
The classification in Fig. 1 is based on the 18 items of DTx Product Evaluation Considerations of DTx-Value-Assessment-Guide. For details of each item, please refer to DTx-Value-Assessment-Guide [Note 1]
2. 6 elements of product success
In addition to the above basic requirements for product listing, according to [Note 3], it proposes six key factors to ensure the success of DTx products: Interoperability, Socialization, Outcomes, Engagement ), Intelligence, and Integration.
Interoperability , using advanced technologies to allow sharing of data across multiple platforms.
Intelligence leverages advanced technologies such as machine learning, deep learning and artificial intelligence to generate personalized, evolving systems and interventions.
Socialization allows the exchange of cumulative or real-time patient data with other member affiliates.
Integration involves using DTx products as part of a patient's lifestyle, spending more time managing their own care and less time with clinicians.
In addition, Outcomes , from preliminary studies to randomized controlled trials, are important in establishing a successful DTx product.
Finally, gaming principals should be applied to DTx interventions to satisfy engagement .
From all these factors, interoperability should be the focus as it enhances big data processing, communication, qualified research and international cooperation [Note 2]
Therefore, it can be seen that compared with general medical materials or drugs, these products emphasize the integration and interaction with users. Whether it is the use of artificial intelligence to respond, it will be combined with the assistance of medical care providers. It is foreseeable that the service will occupy the important weight.
In addition, if it is sold across countries and encounters regions with different language families, the effectiveness of Linguistic Validation, which has recently been valued by medical material regulations, will be an important specification.
Discussion on the role of DTx product development
1. Ideal participating manufacturers
According to Deloitte's Crossing the Boundary - Exploring the Strategies of Digital Medical Regulations[Note 4], the digital medical device ecosystem can be represented as Fig2.
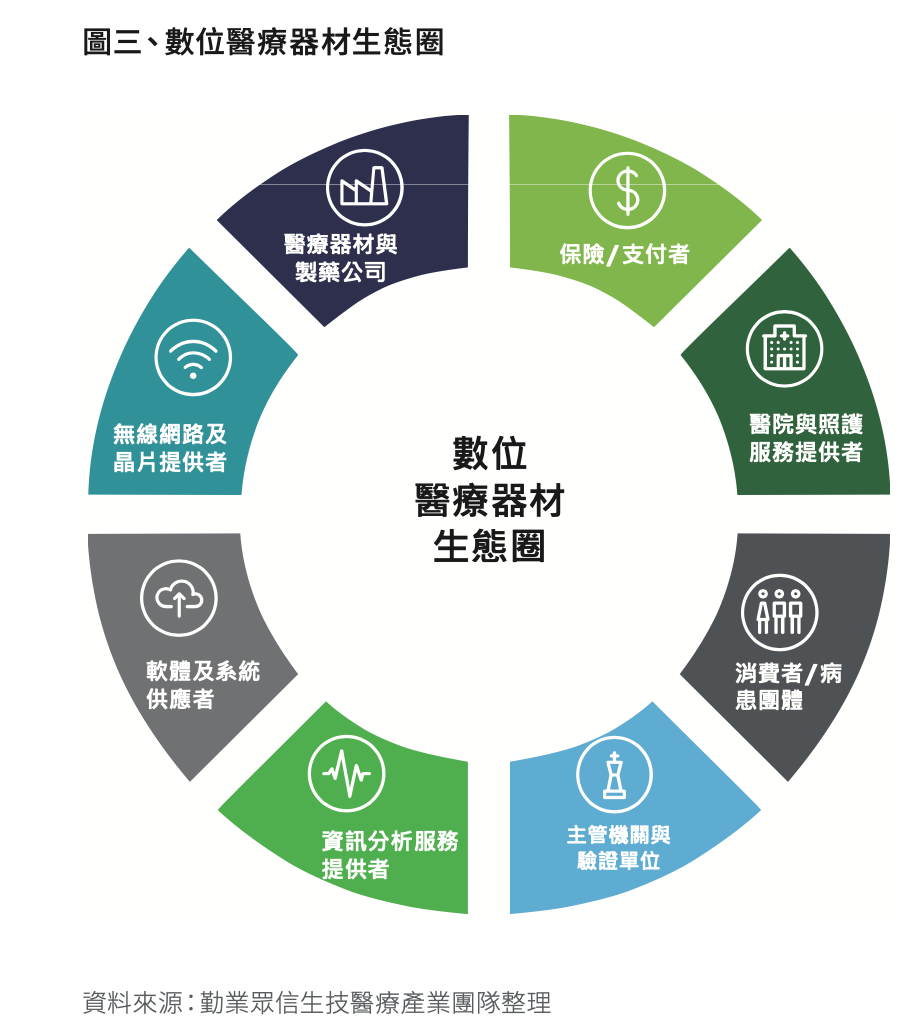
The ideal Iron Triangle of DTx developers is medical devices, pharmaceutical companies, and information analysis service providers as shown in Figure 3.
From the perspective of marketing approval, the ideal of combining medical devices and pharmaceutical companies lies in the complementarity of laws and regulations, because medical materials and drugs are different legal norms, and the characteristic of DTx is that the relevant product regulations are mainly SaMD, that is, medical materials. However, the method of clinical trials is closer to that of drugs.
For the qualification of medical material manufacturers, their products require long-term third-party research and reference materials for other applied research such as observational studies . Otherwise, a medical material without sufficient clinical research support cannot guarantee the results of clinical trials. believable.
For post-market tracking and even customer service, information analysis service providers play the most critical role.
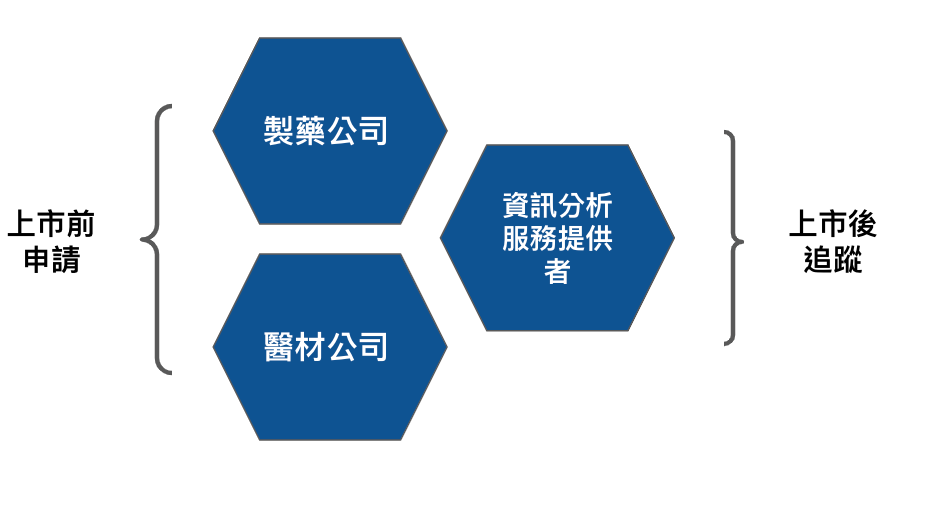
In addition, if it is a behavioral therapy ( CBT, Cognitive behavioral therapy ), in addition to the pharmaceutical company matching the medical equipment, the combination of the medical center and the medical equipment company is also another option to consider. This combination has another advantage. Directly leverage healthcare provider resources.
It can be seen that DTx is actually a product that requires cross-industry integration.
2. Organizational realization
Because the basic requirement structure of the regulations is similar to the US FDA's Pre-Cert Program or AI/ML Framework, for the discussion of relevant specifications, please refer to the following column
The requirements of the relevant laws and regulations are included, in addition to the description of the relevant structure, and finally the requirements of the laws and regulations are explained in the form of specifications.
As for the realization of the organization, the author proposes the following design for the relevant organizations according to the requirements of the above regulations
In addition, in terms of organization, because it is an organization that requires cross-industry integration, and a task organization formed by medical equipment, pharmaceutical companies, and information analysis services, because this is a major in medical regulations, the leader will be medical equipment or pharmaceutical company.
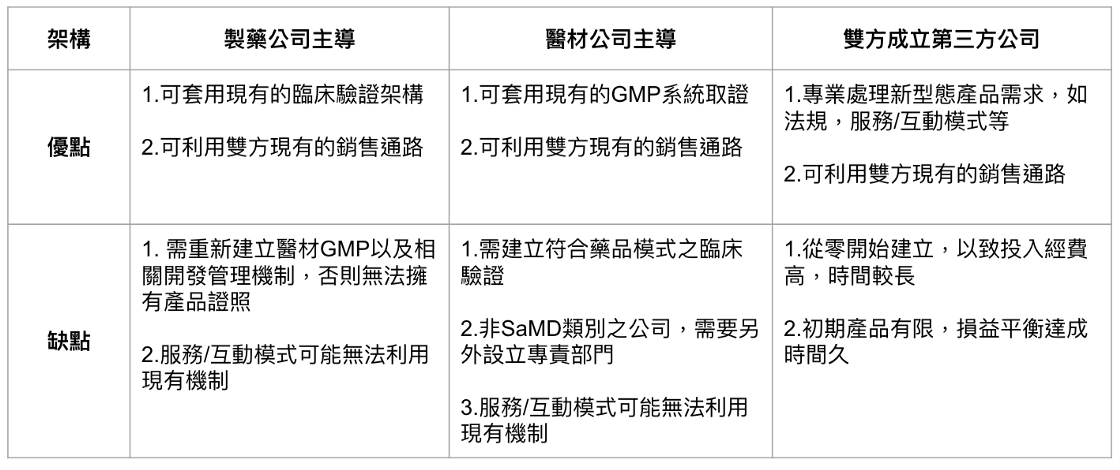
(1) Leading pharmaceutical companies, integrating medical material companies and information analysis service companies: The advantage is that digital therapy can be regarded as a kind of drug, so in clinical validation, it can be done with ease, and it may be that pharmaceutical companies are the most suitable for some treatments. Production of digital therapy content.
But the potential disadvantage is that if the pharmaceutical company does not have a separate GMP system for medical materials, then in theory, the regulations need to be applied by the medical material company, because the digital therapy is SaMD, and the software is the medical material category.
(2) Leading the medical material company and integrating the information analysis service company of the pharmaceutical company: The advantage lies in the direct use of the GMP system of the medical material company to develop products, and the pharmaceutical company is the content provider.
However, the potential disadvantage is that in terms of clinical validation, the existing model of medical material companies may not be applicable, and it needs to rely on the assistance of pharmaceutical companies.
In addition, if the medical material company is not a SaMD-based company, it has to set up another unit dedicated to SaMD in organization. Although it can be included in the current Notify Body category, whether the extra business unit is suitable for the current operation and whether it will cause resources squeeze out? This is an issue that needs to be considered.
(3) A joint venture between a pharmaceutical company and a medical material company to set up a new company: the advantage is that the division of labor does not affect the current organizational management, and the disadvantage is that additional resources need to be invested.
In addition, the cross of when a new company or a new business can break even is another consideration.
The three methods have their advantages and disadvantages, as shown in Fig. 4, depending on the resources of both parties.
discuss
All in all, companies that want to realize digital therapy products are organizations that combine the characteristics of medical devices, pharmaceutical companies, and information analysis services.
Although it is a type of drug, GMP is the structure of medical materials. In addition, post-market tracking and in-depth user interaction are also important parts.
From the author's point of view, because this is a new type of product, the existing ecosystem does not have a completely suitable organization, so it is a more reasonable approach to set up a dedicated company.
As for the challenge of profit and loss balance, in practical operation, it may be possible to consider looking for a medical material company that mainly focuses on SaMD products, or a company with software such as Teleheath and Remote Monitoring as the backbone.
As far as this company is concerned, the resources required to join the DTx product are far lower than the re-establishment of a pharmaceutical factory or the establishment of a new business by a hardware and medical materials company, and even the operational efficiency is much higher.
Note 1: https://dtxalliance.org/advancing-dtx/dtx-value-guide/
Note 2: Accelerating Biopharmaceutical Innovation With Digital Therapeutics To Improve Patients' Quality Of Life
The Biofourmis Therapeutics Approach | Advancing Drug Development & Efficiencies
Note 3: Hong, JS, Wasden, C., & Han, DH (2021). Introduction of Digital Therapeutics. Computer Methods and Programs in Biomedicine, 106319
Note 4: Crossing Borders - Exploring Digital Healthcare Regulatory Strategies
Like my work?
Don't forget to support or like, so I know you are with me..
Comment…