The status quo and trend of the development of bacteria in the treatment of tumors
The rapid development of modern medicine is still unable to overcome the incurable problem of most advanced tumors, which has greatly promoted the development of "unconventional" treatment methods such as viruses, bacteria and malaria parasites. In particular, the method of treating tumors with a mixture of bacteria invented by Dr. Coley in the 1890s has once again become a research hotspot in the 21st century due to the simple and easy operation of bacteria.
The most researched tumor-targeting bacteria include Clostridium novyi-NT, Salmonella typhimurium, Bifido-bacterium, E. coli Nissle 1917, etc., which are often used Tumor treatment. The anti-tumor engineering bacteria constructed based on the above bacteria can be treated alone, combined with chemotherapy, radiotherapy and immunotherapy to inhibit or kill tumor cells. At the same time, tumor-targeted preparations prepared by a variety of bacteria and their derivatives have entered clinical trials or are undergoing relevant clinical recruitment. With the development of bioomics technology, as well as the advancement of gene modification technology and the rise of immunotherapy, a new development trend has emerged in the treatment of tumors by bacteria. This article summarizes the latest research progress in the treatment of tumors by bacteria, and discusses its development trends, application prospects and existing problems.
1 Bacteria's targeting of tumors
1.1 Solid tumor microenvironment
The solid tumor microenvironment is the basis for the design and application of bacterial targeted therapy for tumors. Solid tumors are different from the normal tissues of the body. They have the characteristics of low oxygen, low nutrition, low pH and high permeability. In the process of increasing volume of solid tumors, the local blood vessels of normal tissues are insufficient to maintain the growth of tumor cells, which triggers the release of tumor angiogenesis factors to form a new vascular network and lose the normal vascular structure. This chaotic and unstable network of neovascularization is mainly distributed around the tumor tissues, making the supply of oxygen and nutrients in the tumor insufficient, causing temporary or persistent hypoxia at the tumor site, and generating necrotic tissue at the same time. In order to cope with hypoxia, tumor cells will supply energy through glycolysis and anaerobic respiration, but will produce lactic acid, making the pH of the tumor site lower than normal tissues. The dense extracellular matrix and higher cell density of solid tumors result in a higher osmotic pressure inside the tumor, which prevents drug molecules from penetrating into solid tumors lacking blood vessels. Due to the particularity of the microenvironment of solid tumors, the current clinical treatment methods are less effective. Therefore, we urgently need to find new and efficient tumor treatment methods.
1.2 The characteristics of bacteria and their ability to target tumors
In nature, some bacteria can specifically target solid tumors, overcome the low oxygen and high osmotic pressure of solid tumors, and enter the necrotic area of tumors to grow and multiply. For example, attenuated Novibacterium and Bifidobacterium prefer anaerobic environment, they will automatically gather at the tumor site after intravenous injection, but cannot survive in normal tissues, which effectively avoids the damage of bacteria to normal tissue cells. Under normal circumstances, the drug can only passively flow with the blood, causing the concentration of the drug to reach the tumor to decrease correspondingly as the distance increases. At the same time, due to the lack of blood vessels inside the tumor and the high osmotic pressure microenvironment, the drugs cannot enter the tumor.
Bacteria, as organisms, are targeted to accumulate in tumors under the action of chemokines and enhanced permeabil-ity and retention effect (EPR), and enter the solid tumors to grow and multiply by virtue of their flagellar movement. Some studies have shown that whether Salmonella typhimurium is in vitro or in vivo, its genetic material remains stable after multiple generations of reproduction. This lays a solid foundation for the construction of tumor-targeting engineering bacteria. DNA recombination technology can be used for tumor-targeting bacteria. Genetic modification produces bacterial strains with higher tumor targeting and less toxicity for targeted tumor therapy.
In fact, facultative anaerobes are less targeted to tumors than obligate anaerobes, but their convenience for modification in vitro makes them a major research object. Genetic modification can effectively improve the tumor targeting of facultative anaerobes. For example, placing the gene asd necessary for the growth of Salmonella typhimurium strains behind the hypoxic promoter PpepT makes the strain can only grow and reproduce in a hypoxic tumor environment, and when in aerobic normal tissues, aerobic activation The daughter PsodA promotes the antisense transcription of the asd gene, making it unable to survive in normal tissues (Figure 1).
More importantly, bacteria can not only enter the solid tumor, but also metastasize with tumor cells. After Salmonella typhimurium was injected intravenously into mice, the bacterium appeared in every metastasis of the tumor. Among them, 44.0% of bacteria are scattered in tumor metastasis tissues, while only 0.5% of bacteria are present in normal liver tissues. In addition, αvβ3 integrin is usually highly expressed on activated endothelial cells and some tumor cell membranes, and arginine-glycine-aspartic acid (RGD) is highly targeted to it. Using DNA technology to express RGD polypeptide on the cell membrane of Salmonella typhimurium can significantly increase its tumor targeting (Figure 1).
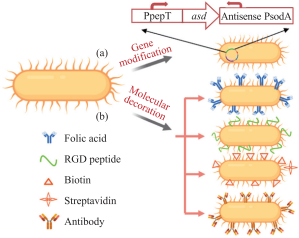
Fig.1 Increasing bacterial tumor-targeting ability using gene modification and molecular decoration
(A) Genetic modification. The essential gene (asd) is expressed under the control of the hypoxia promoter (PpepT), and the aerobic promoter (PsodA) will promote the antisense transcription of the asd gene; (b) by modifying small molecules, peptides and antibodies on the surface of bacteria. Targeting molecules increase their tumor targeting.
(a) Gene modification. The hypoxic microenvironment of solid tumor activates hypoxia promoter(PpepT) to enhance the expression of essential gene
(asd)while aerobic promoter(PsodA) promotes antisense transcription of asd gene and silences the gene in the normal organs; (b) Modification of
bacterial membrane with small molecules, peptides, antibodies and other tumor-targeting molecules for enhancing their tumor-targeting ability.
In addition, expressing carcinoembryonic antigen-specific antibodies on the surface of bacteria can not only increase the tumor targeting of bacteria, but also significantly enhance the immunogenicity of cancer vaccines based on bacteria. The synthesis and expression of adhesin through tumor-targeting bacteria can significantly increase the adhesion of bacteria to solid tumors, thereby improving the tumor-targeting properties of bacteria. In addition, in view of the characteristics of the tumor’s acidic microenvironment, genetically modified Salmonella typhimurium enables the STM1787 promoter to be preferentially activated in the acidic tumor microenvironment, thereby driving the expression of therapeutic genes, and using Salmonella to specifically target the primary tumor and The characteristics of metastases in the body achieve the purpose of treatment.
In addition to using the characteristics of low oxygen and low pH of the tumor microenvironment to modify bacteria, designing auxotrophic bacteria is also a common method to improve bacteria targeting tumors. The Salmonella typhimurium A1 strain is designed as a leucine/arginine auxotrophic bacterium, which can obtain sufficient nutrition in the tumor and colonize the tumor site, but it cannot grow normally due to the lack of essential amino acids in normal tissues . Lipopolysaccharide is an important component leading to bacterial toxicity. The deletion mutant of Salmonella typhimurium that knocks out the lipopolysaccharide gene has good tumor specificity and significantly reduced pathogenicity.
2 Bacteria use nanomaterials and their derivatives to target imaging and treat tumors
Nanomaterials have good tumor permeability, EPR effect and high drug loading capacity, which makes nanomaterials have important application potential in tumor treatment. Nanomaterials have a small particle size that allows them to traverse capillaries freely. The EPR effect can make the nanomaterials stay in the tumor site more, but the liver and spleen will trap more than 70% of the nanoparticles. In order to enhance the tumor-targeting ability of nanoparticles, tumor-targeting molecules can be modified on the surface of the nanoparticles, such as antibodies, affinities, peptides or small organic molecules of receptors on the surface of tumor cells.
However, the purification and preparation costs of these targeting molecules are relatively high, the modified components are unstable, and only some particles can reach the tumor site. The unique tumor-targeting properties of bacteria can make up for the deficiencies of nanomaterials in tumor treatment. For example, researchers use Clostridium difficile (Clostridium difficile) spores to carry nanomaterials, and combine drugs with nanomaterials through antibodies against the nanomaterials. The spores of Clostridium difficile cannot grow and reproduce under aerobic conditions, and can germinate and grow only when they reach the hypoxic tumor microenvironment. This antibody-oriented strategy has shown a better therapeutic effect.
In addition, nanomaterials are introduced into bacteria by electroporation, and folic acid is embedded on the surface of bacteria so that it can bind to the folic acid receptors highly expressed on the surface of tumor cells to achieve the purpose of targeting tumors. More drugs are delivered to tumor cells (Figure 1). In order to improve the efficacy and specificity of tumor treatment, low-boiling perfluorohexene is wrapped in nanoparticles combined with bifidobacteria, and high-intensity focused ultrasound is applied to the outside to destroy the cells after the liquid-gas phase transition to eliminate tumors. At the same time, cationic polymers are used to wrap DNA to form cationic nanospheres, which are adsorbed on the surface of Salmonella typhimurium by electrostatic interaction, so as to achieve the purpose of immunotherapy of tumors by targeted bacteria carrying DNA vaccines.
In addition, the bacteria secrete out-er membrane vesicles (OMV) during the growth process, which are formed by the protruding outer membrane of the bacteria, and carry the cytoplasm of the bacteria and the material in the periplasmic space. Most of the vesicles are wrapped with lipopolysaccharides. This small-sized micro-bacteria can strongly activate the host's immune response. Some researchers knocked out the msbB gene in the E. coli W3110 strain to inhibit the synthesis of lipopolysaccharide, which can significantly reduce the toxicity of bacterial vesicles.
The DNA drug is bound to the nanoparticle, and the bacterial vesicle is used to carry it into the tumor site. After being swallowed by the tumor cell, the DNA drug is released and can be expressed in the cell. Using electroporation technology to introduce spindle kinesin siRNA into affibody OMV expressing HER2 (human epidermal growth factor receptor 2), which can effectively reduce the expression of spindle kinesin in tumor cells, block the cell cycle and induce cell apoptosis Death. Adoptive transfer and gene knockout studies have confirmed that the immune protection of OMV produced by Escherichia coli is mainly through stimulating T cell immunity and inducing Th 1 and Th 17 cell responses, thereby effectively reducing the lethality induced by bacterial infection. Knock out the cell cycle regulating gene minCD of bacteria, causing cells to divide abnormally and produce more bacterial vesicles. At the same time, displaying the acidic microenvironment targeting polypeptide on the surface of the bacteria can make the bacteria target the tumor acidic microenvironment and improve the targeting of microcells to tumors.
In addition, bacterial nano-level derivatives, such as nano-magnetosomes produced by magnetotactic bacteria, have been widely used in tumor imaging and treat. The anti-HER2 affibody membrane anchoring protein MamC was co-expressed through genetic recombination, and the HER2 targeted nano-magnetosome was prepared by using the interaction of MamC and the phospholipid bilayer to embed the affibody on the membrane surface of the nano-magnetosome.
This engineered nano-magnetosome can be combined with HER2 overexpressed on the surface of tumor cells to achieve targeted aggregation and magnetic resonance T2 imaging of the nano-magnetosome at the tumor site. If the nanomagnetosomes are injected into the skull of mice bearing gliomas, under the action of an alternating magnetic field, the nanomagnetosomes will generate heat to kill tumor cells, and 40% of the mouse tumors will disappear completely. At the same time, magnetically guided tumor targeted therapy based on the magnetic guidance of magnetotactic bacteria has emerged. For example, each cell in Magnetococcus marinus MC-1 contains a magnetic iron oxide nanocrystal chain, which moves along the local magnetic field lines. Based on the two-state pneumatic sensing system, it swims in the direction of hypoxia and can be distributed in the tumor. Even, the drug-containing nano-liposomes can be used to carry the drug, which can significantly increase the concentration of the drug in the hypoxic area of the tumor.
3 Bacteria combined with chemotherapy, radiotherapy and immunotherapy to treat tumors
3.1 Targeted treatment of tumors with chemotherapeutic drugs carried by bacteria
Chemotherapy is the most important treatment method for metastatic cancer, because drugs can follow the blood flow to reach various metastases throughout the body, thereby performing systemic treatment. However, due to the higher interstitial internal pressure and less blood vessel distribution in solid tumors, drugs cannot enter the tumor. At the same time, chemotherapy drugs not only kill tumor cells, but also affect the physiological functions of normal cells such as hair follicles, bone marrow, and gastrointestinal tract, and induce serious toxic and side effects in the body.
Increasing the tumor targeting of chemotherapeutics can effectively reduce the damage to normal tissue cells. Many studies have proved that bacteria can carry chemotherapeutic drugs to achieve targeted treatment of tumors and reduce the side effects of drugs on the body. For example, liposomes (lipidosome) encapsulated paclitaxel (pacli-taxel) drugs, relying on streptomycin (streptomycin) combined with the biotin (biotin) of the bacterial outer membrane, can promote the accumulation of paclitaxel at the tumor site (Figure 2).
In addition, temperature-sensitive liposomes are designed to encapsulate doxorubicin drugs, and biotin-streptavidin is used to bind the liposomes to the cell membranes of Salmonella typhimurium, and the tumor-targeting properties of Salmonella typhimurium More chemotherapeutic drugs are delivered to tumors, and high-intensity focused ultrasound is used for heating to induce the release of drugs, which can effectively inhibit tumor growth. In the mouse melanoma model, compared with cyclophosphamide alone, the combined treatment of Salmonella typhimurium VNP20009 and cyclophosphamide can significantly reduce the tumor microvessel density and the content of vascular endothelial growth factor. Therefore, compared with chemotherapy alone, bacterial adjuvant chemotherapy can not only enhance the therapeutic effect of tumors, but also reduce the side effects of chemotherapy.
3.2 Bacteria combined with radiotherapy to treat tumors
Radiotherapy is mainly to induce DNA damage of tumor cells to kill tumor cells, and the low oxygen microenvironment in solid tumors will significantly reduce the efficacy of radiotherapy. In addition, studies have shown that radiotherapy can cause protective autophagy in tumor cells, thereby maintaining the stability of the internal environment and allowing tumors to continue to proliferate and metastasize. The radioisotope 188Re was combined with Listeria monocytogenes through an antibody, and injected into mice bearing highly metastatic pancreatic cancer. The isotope is only present in a small amount at the primary site of the tumor, and a large amount in the metastasis ( figure 2). Through starvation reaction, 32P is infiltrated into Listeria and injected into tumor-bearing mice. Listeria 32P can synergistically kill tumor cells at the tumor site through induced ionizing radiation and bacteria-induced reactive oxygen species.
3.3 Bacteria combined immunotherapy to kill tumor cells
As a new type of clinical treatment, immunotherapy has gradually become an important means of cancer treatment. Studies have shown that many patients have experienced grade 3 or 4 adverse events after receiving immunotherapy, including liver, gastrointestinal, and kidney diseases, which can also cause the body to develop immune tolerance. Cancer cells can evade the body's immune system by secreting immunosuppressive cytokines, recruiting immunosuppressive cells, and presenting immune checkpoint proteins. In fact, microorganisms have a wide range of effects on the body's immune system. They can not only regulate the maturation and function of the resident immune cells in the central nervous system, but also affect the activation of peripheral immune cells. Using bacterial expression of flagellin and tumor antigens to co-present to T cells to achieve the optimal antigen presentation effect, promote the activation of CD4+ and CD8+ T cells, and stimulate the body to produce an adaptive immune response to inhibit tumor growth (Figure 2). Using gene modification technology to encode and express CD47 and other nanobody antagonists in Escherichia coli, increase the activation of tumor-infiltrating T cells, make tumors quickly regress, and effectively inhibit tumor cell metastasis.
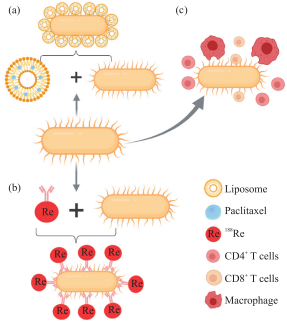
Fig.2 Bacteria combined with other methods to treat tumor
(A) Bacteria encapsulate paclitaxel and other chemotherapeutics to treat tumors; (b) Bacteria cooperate with radioactive elements to eliminate tumors; (c) Bacteria stimulate the body to produce an immune response to inhibit tumor growth.
(a)Bacteria loaded with paclitaxel or other chemotherapeutic drugs for tumor treatment;(b)Bacteria being synergistic with radioactive elements to
eliminate tumors;(c)Bacteria stimulate immune response to suppress tumor growth.
4 Summary and Outlook
Tumor-targeted bacteria can specifically enter cancer in situ and metastases throughout the body, and can grow and multiply in necrotic areas. This unique growth and reproduction method can effectively overcome the shortcomings of existing clinical tumor treatment methods, including chemotherapy, molecular targeted therapy, cell therapy and antibody therapy and other drugs cannot enter the tumor, as well as radiotherapy, surgical treatment and other physical therapy methods. Locate potential cancer metastases, etc. However, the results of some clinical trials that have been completed show that the use of bacteria alone to treat tumors has not achieved significant anti-tumor effects.
This is mainly because the cytotoxicity of tumor-targeted engineering bacteria cannot completely kill tumor cells, and the secreted and expressed drugs cannot spread to all tumor cells of solid tumors. In addition, the growth and reproduction of some bacteria may promote the growth and metastasis of tumors, which has also become a major obstacle to clinical application. Therefore, the cross-complementation of the advantages of bacterial targeted therapy and the existing clinical treatment methods is the only way for it to eventually go to the clinic. In addition, the rapid development of microbiome has profoundly revealed the relationship between the immune interaction of bacteria and the tumor microenvironment. Most of the bacteria that have been transformed into immune stimulating factors have performed comprehensive treatment of tumors alone or in combination with radiotherapy and chemotherapy, and have achieved significant therapeutic effects.
At the same time, the development of nanotechnology provides new auxiliary materials for bacterial targeted therapy, which not only enhances the tumor targeting of bacteria, but also significantly enhances its anti-tumor effect. In recent years, tumor immunology has fully revealed the complex relationship between tumor occurrence and development and the body's immune system, and also indicated that cancer treatment is a systematic project.
As an important supplement to specific immunity, non-specific immunity has great application prospects in the treatment of tumors. In this situation, a number of emerging innovative biotechnology companies have also emerged in the industry. As a Hong Kong drug oncolysis that developed the world's first salmonella drug delivery bacterial carrier YB1, it has also recently attracted widespread attention in the industry with its breakthrough innovative technology research results.
As a scientific and technological innovation R&D company focusing on the development and application of biomacromolecule drug delivery systems, we are using the company’s core technology product-macromolecular drug delivery carrier YB1 to develop YB1 oncolytic bacterial therapy and application in the field of cancer immunotherapy. Thrombolytic bacterial therapy used in the field of antithrombotic therapy. The company’s research has proved that YB1 has a very powerful delivery capability and can efficiently deliver a variety of macromolecular anti-cancer drugs, such as protein drugs, mRNA vaccines, antibody drugs, oncolytic viruses, etc.; and can carry various thrombolytic therapy drugs for Treat many types of thrombotic diseases.
Judging from the experimental research results of the R&D team, YB1 has strong technical compatibility, which can be compatible with chemical drugs, immune checkpoint antibodies, CAR-T and other cell technologies, increasing efficacy and diversified product pipeline design. At present, we have laid out multiple pipelines of products under development for the application of YB1, including 7 pipelines of YB1 oncolytic bacteria. The main indications cover sarcomas, melanomas and other solid tumors; and 3 pipelines of YB1 thrombolytic bacteria, respectively. YB1 carries recombinant urokinase (rt-PA), YB1 carries recombinant defibrinase Defibrinogenase and YB1 carries plasmin (plasmin) thrombolytic drugs under development. The indications are various thrombotic diseases. With the gradual maturity of YB1 technology and more and more outstanding and innovative biotechnology companies in the industry gradually moving towards a broader market, the global biotechnology and medical and health fields will usher in a new atmosphere. We look forward to a new round in the global biotechnology field. The industry has exploded.
喜欢我的作品吗?别忘了给予支持与赞赏,让我知道在创作的路上有你陪伴,一起延续这份热忱!
