
123
With the growing burden of cardiovascular disease, YB1's innovative thrombolytic therapy technology opens the path to life for patients.
With the rapid socio-economic development in China, the lifestyle of the population has undergone profound changes, especially the accelerated aging and urbanization of the population, which has led to a clear trend in the prevalence of cardiovascular disease risk factors in China, resulting in a continuous increase in the number of cardiovascular disease cases in recent years
The number of people suffering from cardiovascular disease will continue to proliferate over the next decade, and cardiovascular deaths now account for the highest number of deaths among urban and rural residents in China. The increasing disease burden of cardiovascular disease in China has become a significant public health problem, and it is urgent to strengthen the prevention and treatment efforts. Innovative treatment technologies have become highly relied upon.
330 million people with cardiovascular disease, generating a market of over $ 200 billion.
Official data from the National Cardiovascular Centre shows that the prevalence of cardiovascular disease in China has been on the rise in recent years. According to projections, the current number of people suffering from cardiovascular disease in China is 330 million, including 13 million people suffering from stroke, 11 million from coronary heart disease, 5 million from pulmonary heart disease, 8.9 million from heart failure, 2.5 million from rheumatic heart disease, 2 million from congenital heart disease, 45.3 million from lower limb artery disease and about 245 million from hypertension.
Cardiovascular disease is the collective term for cardiovascular and cerebrovascular diseases and refers to ischaemic or hemorrhagic diseases of the heart, brain, and body tissues caused by hyperlipidemia, thick blood, atherosclerosis, and hypertension.
As mentioned above, China has the highest mortality rate for cardiovascular disease. The large population of cardiovascular and cerebrovascular patients with a high mortality rate, and the current trend of rapid growth and individual clustering among low-income groups, is one of the primary healthcare issues in China. However, at the same time, the large patient population and the expanding base also represent a lucrative and promising market.
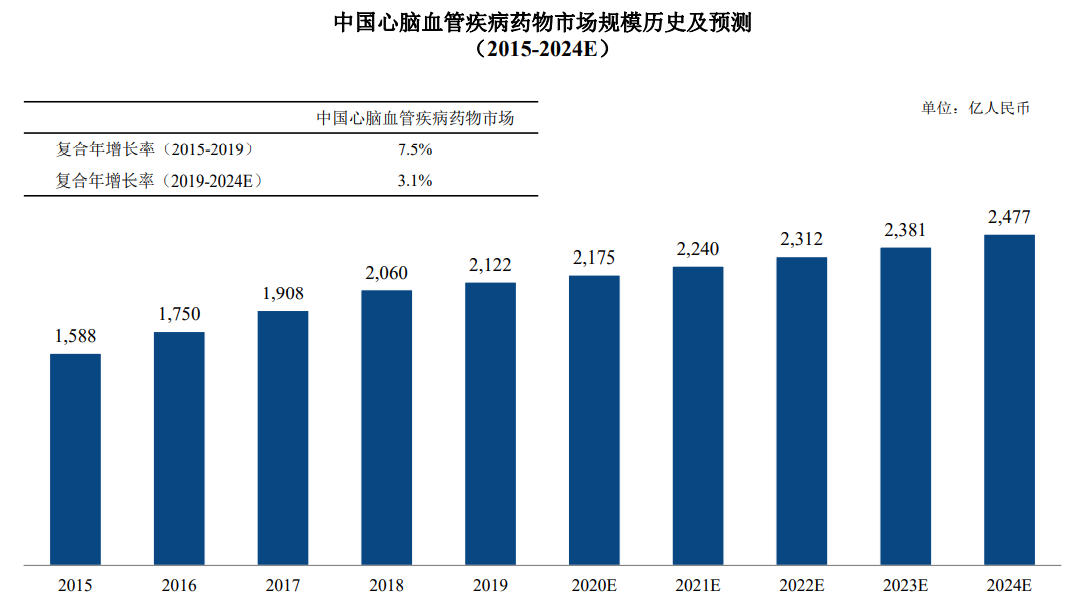
A report by a professional research institute shows that the market size of the cardiovascular and cerebrovascular disease treatment sector will be approximately RMB 212.2 billion in 2019, accounting for approximately 12.99% of the overall size of China's pharmaceutical market. The market size of cardiovascular and cerebrovascular diseases grew from RMB158.8 billion in 2015 to RMB212.2 billion in 2019, with a compound annual growth rate of 7.51%. The agency expects China's cardiovascular and cerebrovascular disease market size to grow to RMB247.7 billion by 2024.
Acute ischaemic stroke - a representative of common cardiovascular diseases
Cardiovascular disease has become a widespread disease in China, and stroke is one of China's most common cardiovascular diseases after hypertension. It can be said that research into the understanding of the development and treatment of stroke is representative of the prevention and treatment of cardiovascular disease.
Stroke is an acute cerebrovascular disease, mainly including ischaemic stroke and hemorrhagic stroke. It is a group of diseases that cause brain tissue damage due to sudden rupture of blood vessels in the brain or blockage of blood vessels causing blood to fail to flow to the brain, and is characterized by high morbidity, mortality, and disability.
Stroke is currently dominated by acute ischaemic stroke (AIS), accounting for about 70.2% of all stroke cases. Publicly available information records that about 4,832,000 people suffered from a stroke in 2019, of which about 3,392,100 suffered from acute ischaemic stroke. Due to the increasing population of hypertension, diabetes, hyperlipidemia, and coronary heart disease, the size of acute ischaemic stroke patients in China is expected to be about 3,977,100 by 2024 and about 4,342,400 by 2030.
In 2019, there were approximately 719,500 deaths due to acute ischaemic stroke in China, and some studies have shown that the death/disability rate of hospitalized acute ischaemic stroke patients within one year of symptom detection ranges from 33.4% to 33.8%.
As the incidence of acute ischaemic stroke continues to rise, it has become a phenomenon in cardiovascular disease, and the treatment of acute ischaemic stroke has become a primary public concern. According to the Chinese Guidelines for the Management of Acute Ischemic Stroke, the primary treatment for acute ischaemic stroke is thrombolytic therapy.
Intravenous thrombolysis is currently one of the most critical measures to restore cerebral blood flow in acute ischemic stroke patients. The drugs commonly used internationally are recombinant tissue-type fibrinogen activator (rt-PA) and tenecteplase (TNK), whereas the drugs currently commonly used in China are rt-PA and urokinase (UK). TNK is currently undergoing clinical validation trials for thrombolysis in cerebral infarction. The currently accepted time window for intravenous thrombolysis is within 4.5 hours of onset or 6 hours.
The time window for treating acute ischaemic stroke is essential because of the lack of blood supply to the brain and the resulting brain damage after treatment for acute ischaemic stroke, which leads to functional impairment.
In 2019, the number of patients with acute ischaemic stroke who can receive thrombolytic therapy in China was about 1,307,700, and the number of patients who can be treated with thrombolytic drugs within six hours of onset is about 967,700, of whom about 758,500 can receive thrombolytic therapy between 4.5 hours and 6 hours. With the future increase in medical resources, the construction of stroke centers, and other favorable policies, it is expected that the number of patients with acute ischaemic stroke who can receive thrombolytic treatment within 6 hours will increase to 1,249,600 in 2024.
YB1 accelerates the development of new antithrombotic drugs in the highly competitive innovative thrombolytic drug development track
According to Frost & Sullivan's analysis, the market size of thrombolytic drugs in China in 2019 is approximately RMB 2.004 billion. At present, the most significant single product of thrombolytic drugs in China is alteplase (rt-PA), accounting for about 70.23% of the market size of thrombolytic drugs in China, with sales scale of about RMB 1.407 billion. The second-largest class of thrombolytic drugs is urokinase, accounting for 14.67% of the market size of thrombolytic drugs in China, with a sales volume of about 294 million yuan. One of the giants in the thrombolytic drug industry, Puyuk, the core product of Tianshi Li, is the third-largest thrombolytic drug product in China, accounting for about 12.24% of the market size of thrombolytic drugs in China.
However, due to the short half-life of recombinant urokinase and rt-PA, which do not relieve the onset of acute thrombosis, patients and healthcare providers expect more new drugs or therapies to relieve and treat acute thrombosis effectively. Furthermore, the industry has welcomed biotech companies to compete together in the R&D track of innovative thrombolytic drugs.
The oncolytic bacterium YB1 developed by HKND can carry the lysozyme rt-PA to accumulate at the thrombus site and be released at a fixed point, solving the problem of its short half-life. It is also a very innovative and cutting-edge attempt in antithrombotic therapy.
Our core product, the tumour oncolytic bacterium YB1, uses the world's first tumour hypoxia-specific targeting technology to deliver a wide range of therapeutic agents, including antibodies, mRNAs, and proteins, as carriers targeting and releasing them to the tumour center to achieve a tumourolytic effect. The combination with urokinase for treating various thrombotic diseases is an essential technological expansion of YB1 beyond tumour immunotherapy.
It is our first generation of targeted thrombus ablation products. The main feature is that urokinase is carried by YB1 and released at the location of the thrombus, which can achieve rapid, targeted release of thrombolytic drugs. Urokinase is produced in the human body by the kidneys and directly activates the conversion of fibrinolytic enzymes into fibrinolytic enzymes, which in medical terms is the first generation of natural thrombolytic drugs derived from urine.
Our other two pipelines of thrombolytic drugs under development are YB1-rt-DE and YB1-rt-PL, which combine fibrinolytic enzymes and fibrinolytic enzymes, and we hope to achieve more new results in the development process soon.
喜欢我的文章吗?
别忘了给点支持与赞赏,让我知道创作的路上有你陪伴。
发布评论…