
123
What are the pain points of traditional thrombolytic therapy? Demand is driving the market, and innovation in thrombolytic drug iteration is urgent.
There was a time when we did not know anything about "stroke".
In the general perception of the younger generation, "stroke" is an outright disease of the elderly, but in recent years, the incidence of stroke, the legendary "disease of the elderly", has not only increased gradually but has even shown a younger trend. In recent years, the incidence of stroke has increased and has even become more prevalent at a younger age, and this, combined with the frequent dissemination of information about brain attacks and sudden deaths on the internet, has made the younger generation aware of the horrific disease.
Stroke generally referred to as a stroke, is commonly known as a stroke or cerebral infarction. Once a stroke occurs, if not detected and treated in time, it can leave severe lifelong disabilities. As one of the major diseases that cause death and disability, the incidence of stroke in China is increasing year by year. National stroke screening data shows that the standardised incidence of first stroke among people aged 40-74 years rose from 189 per 100,000 in 2002 to 379 per 100,000 in 2013, an average annual increase of 8.3%.
Additionally, data on disease burden indicate that in 2016, the incidence of ischemic stroke was 276.75 per 100,000 in China, whereas hemorrhagic stroke was 126.34 per 100,000. Furthermore, the proportion of stroke patients aged <70 years old in China increases, with a trend towards younger patients.
Stroke has now become the number one cause of death in China, with a mortality rate of 126.48 per 100,000 urban residents and 157.00 per 100,000 rural residents in 2017.
Because ischemic stroke accounts for 70% of new strokes, the level of treatment provided for acute ischemic stroke (AIS) has an effect on our population's health and quality of life.
Acute ischaemic stroke is a severe threat to human health as new cases continue to grow.
There are two main types of stroke: ischaemic strokes and haemorrhagic strokes. Acute ischaemic stroke is a condition that occurs when the blood flow through the arteries of the brain is blocked by an embolus (i.e. a large amount of thickened blood). Currently, acute ischaemic strokes account for 69.6% to 70.8% of stroke events in China, and as the population grows and ages, the number of new acute ischaemic strokes will continue to increase.
The following graph shows the change in the incidence of acute ischaemic stroke in the country.
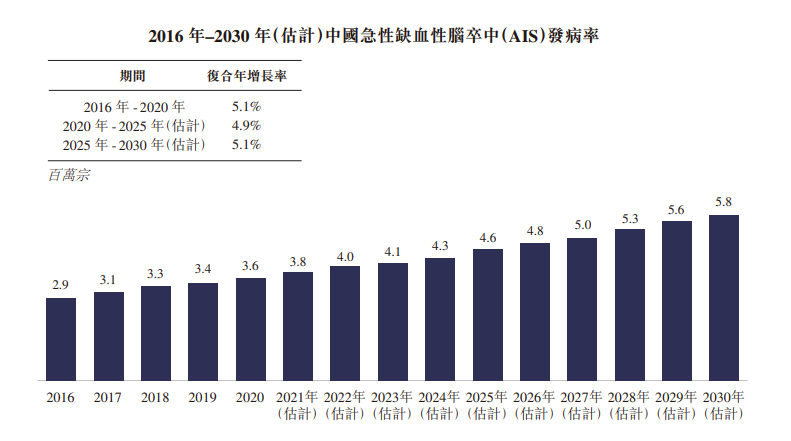
Publicly available information shows that the incidence, disability, recurrence and death rates of acute ischaemic stroke are high and seriously affect human health and life.
Thrombolytic therapy is one of the most effective means of changing the outcome of 'sudden death' in stroke patients.
Intravenous thrombolysis (IVT) and mechanical thrombus removal (MT) are the mainstays of treatment for acute ischaemic stroke, an aggressive intracranial cerebrovascular disease. At present, ultra-early IVT is one of the most effective pharmacological treatments to improve outcomes in acute ischaemic stroke and has been recommended in China and in many national guidelines, but the proportion of acute ischaemic strokes treated with thrombolysis remains low at this stage.
Intravenous thrombolysis uses thrombolytic drugs such as recombinant tissue-type fibrinogen activator (rt-PA) administered intravenously to treat patients with acute ischaemic stroke. It is important to note that intravenous thrombolysis is usually administered within 6 hours of the onset of symptoms and is the standard of care for all eligible patients.
Studies have shown that only about 20% of patients currently reach the emergency department within 3 hours of onset, 12.6% are suitable for thrombolysis, but only 2.4% are treated with thrombolysis, including 1.6% with intravenous thrombolysis with recombinant tissue-type fibrinogen activator (rt-PA).
One of the main difficulties in administering ultra-early thrombolysis for acute ischaemic stroke is that most patients do not arrive at the hospital in time or have in-hospital delays for various reasons. The strict limitation of the six-hour golden treatment window is arguably one of the major pain points in the thrombolysis market and certainly one of the significant difficulties for doctors and patients in their fight against death.
The US and other developed countries have reformed their healthcare systems to improve access to and benefit from thrombolytic therapy for patients with acute ischaemic stroke. It makes thrombolytic therapy "evidence-based".
Common thrombolytic drugs at a glance, innovation and iteration are urgent.
Intravenous thrombolysis is currently one of the most critical measures to restore cerebral blood flow in patients with acute ischaemic stroke, and the most commonly used drugs internationally are recombinant tissue-type fibrinogen activators (rt-PA) and tenecteplase (TNK). The market for thrombolytic drugs has also expanded significantly in recent years, driven by the growing demand.
First-generation thrombolytic agents: Urokinase (UK) - As one of the most commonly used thrombolytic agents in China today, urokinase is well established in the clinical application of thrombolytic therapy.
The trial on "Intravenous thrombolysis with urokinase within 6 hours of acute ischaemic stroke" was divided into two phases: the first phase of the open trial initially confirmed the safety of domestic urokinase. It determined the dose of urokinase to be 1 million to 1.5 million IU. The second phase was a multicentre randomised, double-blind, placebo-controlled trial, which showed that thrombolysis with urokinase (at doses of 1 million IU and 1.5 million IU) was relatively safe and effective in patients with acute ischaemic stroke within 6 hours of onset.
Second generation thrombolytic agent: alteplase (rt-PA) - Currently, several clinical trials have been conducted in the industry to evaluate the efficacy and safety of intravenous thrombolysis with alteplase in patients with acute ischaemic stroke. The latest edition of the Chinese Stroke Prevention and Control Guidelines indicates that the time windows for treatment in studies of alteplase include within 3 hours, 3.0-4.5 hours and 6 hours after onset.
At three months, the National Institute of Neurological Disorders and Stroke (NINDS) trial discovered that complete or near-complete neurological recovery was significantly greater in the intravenous alteplase thrombolysis within three hours group than in the placebo control group. Death rates were comparable in both groups, and the incidence of symptomatic intracranial hemorrhage was significantly greater in the treatment group than in the control group. The results of the European Cooperation Acute Stroke Study III (ECASS III) trial showed that intravenous alteplase remained effective 3.0 to 4.5 hours after onset.
The latest version of the guideline also states that the indications for intravenous alteplase are not yet exhaustive in clinical practice and that, in principle, all patients without contraindications can be treated with intravenous thrombolysis with alteplase. However, this needs to be considered individually due to the variability of the patient's condition.
Third generation thrombolytic agents: Teneprase (TNK) - this is the latest generation of thrombolytic agents. The results of the Attipase - Teneprase Stroke Thrombolytic Therapy Trial Evaluation (ATTEST) and the Norwegian Teneprase Stroke Study (NORTE ST) related studies have shown that teneprase and alteplase have similar thrombolytic effects and safety in patients with mild stroke.
The newly published multicentre randomised controlled study of teneprase versus alteplase prior to endovascular thrombectomy (EXTEND-IA TNK) suggests that teneprase has a faster and better vascular opening capacity than alteplase for acute cerebral infarction in large vessel lesions and could be an effective alternative to alteplase before endovascular treatment.
Short half-life is a pain point for traditional thrombolytic therapy, and new players may stimulate thrombolytic drug iterations.
However, the two major thrombolytic drugs, recombinant urokinase and rt-PA, have a short half-life of 4-8 minutes and do not relieve the onset of acute thrombosis, which is another pain point traditional thrombolytic drug therapy.
As an innovative biopharmaceutical company, the biomacromolecular drug delivery vehicle YB1 developed by HKopharm Lysis can target the release of thrombolytic agent at the location of thrombosis, avoiding the problems caused by excessive thrombolysis, and has recently attracted widespread attention in the industry market.
Our core technology, YB1, uses the world's first thrombus-targeting technology and can be combined with a variety of thrombolytic drugs to precisely target the thrombus and release the thrombolytic drug, exerting a precise and targeted thrombolytic effect.
In terms of specific drug pipeline, YB1-rt-PA is the company's first generation of targeted thrombus ablation product, which is mainly characterised by YB1 carrying and releasing urokinase at the location of the thrombus to achieve rapid, targeted release of thrombolytic drugs. In addition, we have also laid out two product pipelines, YB1-rt-DE and YB1-rt-PL, which combine YB1 with fibrinolytic enzyme and fibrinolytic enzyme. We also look forward to the eventual maturation of this new technology, which will bring more surprises to the thrombolytic therapy market and contribute to the innovation and iteration of thrombolytic drugs.
喜欢我的文章吗?
别忘了给点支持与赞赏,让我知道创作的路上有你陪伴。
发布评论…